MINISTRY OF EDUCATION AND
TRAINING
VIETNAM ACADEMY
OF SCIENCE AND TECHNOLOGY
GRADUATE UNIVERSITY SCIENCE AND TECHNOLOGY
----------------------------
DANG VIET HAU
STUDY ON CHEMICAL CONSTITUENTS,
BIOLOGICAL ACTIVITIES OF CONIFER SPECIES:
DACRYCARPUS IMBRICATUS AND FOKIENIA HODGINSII
Major: Organic Chemistry
Code: 62 44 01 14
SUMMARY OF CHEMISTRY THE DOCTORAL THESIS
HA NOI - 2017
Thesis was completed at:
Institute of Chemistry
Vietnam Academy of Science & Technology
Advisers:
1. Assoc. Prof. Dr. Trinh Thi Thuy
Institute of Chemistry- Vietnam Academy of Science & Technology
2. Dr. Tran Van Loc
Institute of Chemistry- Vietnam Academy of Science & Technology
1st Reviewer: ..............................................................................
2nd Reviewer: .............................................................................
3rd Reviewer: ...............................................................................
The dissertation will be defended at: Graduate University Science and
Technology - Vietnam Academy of Science & Technology- No. 18 Hoang
Quoc Viet - Cau Giay - Hanoi.
At............hour.............day............month............ 2017
Thesis can be found in:
-The library of Graduate University Science And Technology
-National Library of Vietnam

1
INTRODUCTION
1. The urgency of the thesis
Thanks for industrial development, human life is increasingly
improving. However, the downside of this development is environmental
catastrophe. Moreover, abuse of chemicals and medicines plant protection
in agriculture and food industry, together with the resistance of the virus
species, the bacteria, the variation of this species do encounter evidence of
human diseases such as cancer, HIV/AIDS, heart disease, diabetes, SARS,
or recent viral fewers.
To deal with the challenges of constant evolution of life, scientists
must continuously invent new medicines providing selected effects,
effective, and affordable to both human patients and the conservation of
rare plant and animal species. According to scientists, studying the nature
and mimic-nature are the best the way for human being to exist and adapt
to their living environments. One of the approaches is researching new
structures with potential activity that be able to be developed into
medicines for humans, livestock, and crops from natural compounds. Over
millions of years of evolution, these natural compounds have mounted up a
flexible compatibility and relatively suitability with living organisms;
especially, they also contain less toxic and perform morefriendly to
environment.
Located in the tropical monsoon climate, Vietnam has extremely
diverse vegetation and abundant with approximately 13,000 species , of
which 309 branches and 4,000 species can be adopted to make drugs. As a
result, these are invaluable natural resources of the country. Although
there are many types of herbs that can be used to make medicines for
people, livestock and crops, their economic performance is still limited
because the exploit and usage are still based on folk experience.
Based on this scientific background, we chose the project "Study on
chemical constituents, biological activities of conifer species Dacrycarpus
imbricatus and Fokienia hodginsii".
2. The objective of the thesis
- Study on the chemical constituents of the species Dacrycarpus
imbricatus (Blume) de Laub, Fokienia hodginsii (Dunn) Henry a. et
Thomas.
- Investigation in to their biological activities
3. The main content of the thesis

2
- Purification and isolation of compounds from crude extracts of
these species is techniques as column chromatography, flash column
chromatography, etc.
- Determine structure of the pure compounds by the combination of
spectral methods such as IR, MS, 1D-2D-NMR, NMR, etc.
- Some isolated compounds were tested biological activities in order
to discover potential compounds.
Chapter 1: OVERVIEW
1.1. Characteristics of plant and study of the genenus
Dacrycarpus, family Podocarpaceae
1.1.1. Characteristics of plant genenus Dacrycarpus
1.1.1.1. Characteristics of plant species Dacrycarpus imbricatus
1.1.1.2. Characteristics of plant species Dacrycarpus dacrydioides
1.1.1.3. Characteristics of plant species Dacrycarpus vieillardii
1.1.2. Used in traditional medicine
1.1.3. The chemical constituents and biological activity of genera
Dacrycarpus
1.1.3.1. Diterpene compounds
1.1.3.2. Flavonoids glucoside compounds
1.2. Characteristics of plants and study of the genus Fokienia.
Henry & H.H. Thomas, family (Cupressaceae)
1.2.1. Family Cupressaceae (Cupressaceae)
1.2.2. Characteristics of plant of the genenus Fokienia.
1.2.3. Characteristics, distribution of species Fokienia hodginsii
(Dunn) Henry a. H.H & Thomas
1.2.4. Use and application of traditional medicine
1.2.5. The chemical constituents and biological activity of species
Fokienia hodginsii
1.2.6. Chemistry and biological activities of diterpene compounds
Chapter 2: EXPERIMENT
This chaper describes the collection, determination, extraction,
isolation, spectral data, and bioactivity assays of isolated compounds from
these plant materials.
3
2.1. Materials, chemicals and equipment
2.2. Research method
2.3. Extraction and isolation of the compounds from two researched
trees
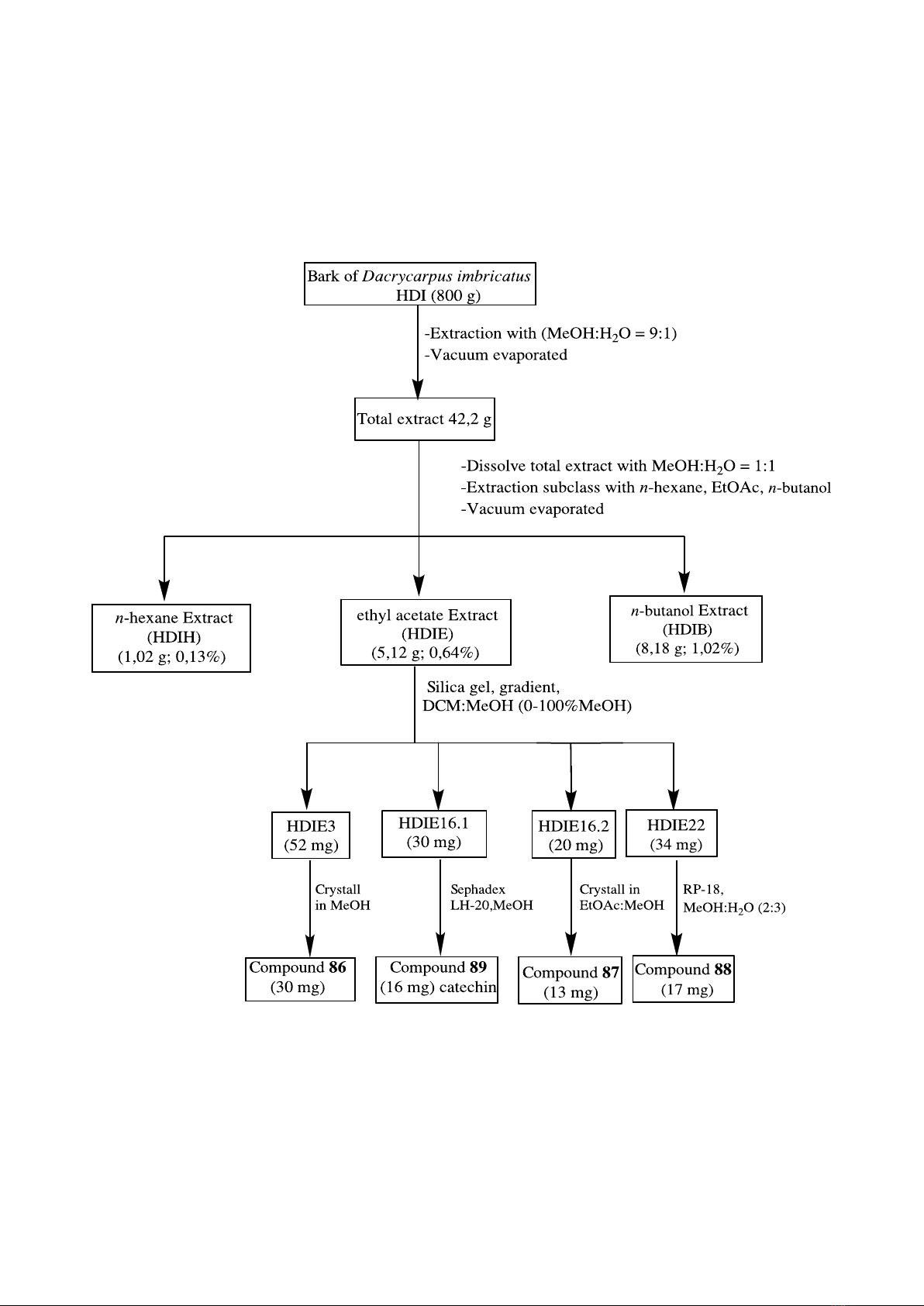
2.3.1. Dacrycarpus imbricatus
Figure 2.1. Schematic diagram showing steps for the isolation of pure
compounds from the bark of Dacrycarpus imbricatus


























