
International University
Biotechnology
Biochemistry
Lecturer: Msc. Le Hong Phu

Topic 9: Detailed
Mechanism of Enzyme
Catalyst
•Group Members:
1. Do Ngoc Anh Huy
2. Tran Van Kha
3. Doan Luong Huy
4. Dang Duong
5. Nguyen Quang Toan
6. Nguyen Thi Huong Giang
7. Pham Vinh Phuoc
Group leader

Content:
•Definition and basic process:
1. Induced fit
2. Mechanisms of transition state stabilization
•Detailed in example to make you
understand more about mechanism of
enzyme catalyst:
Chymotripsin

Lock-key theory
•A Specific active site for a specific substrate
How can make the
key to be fixed to the
locker???
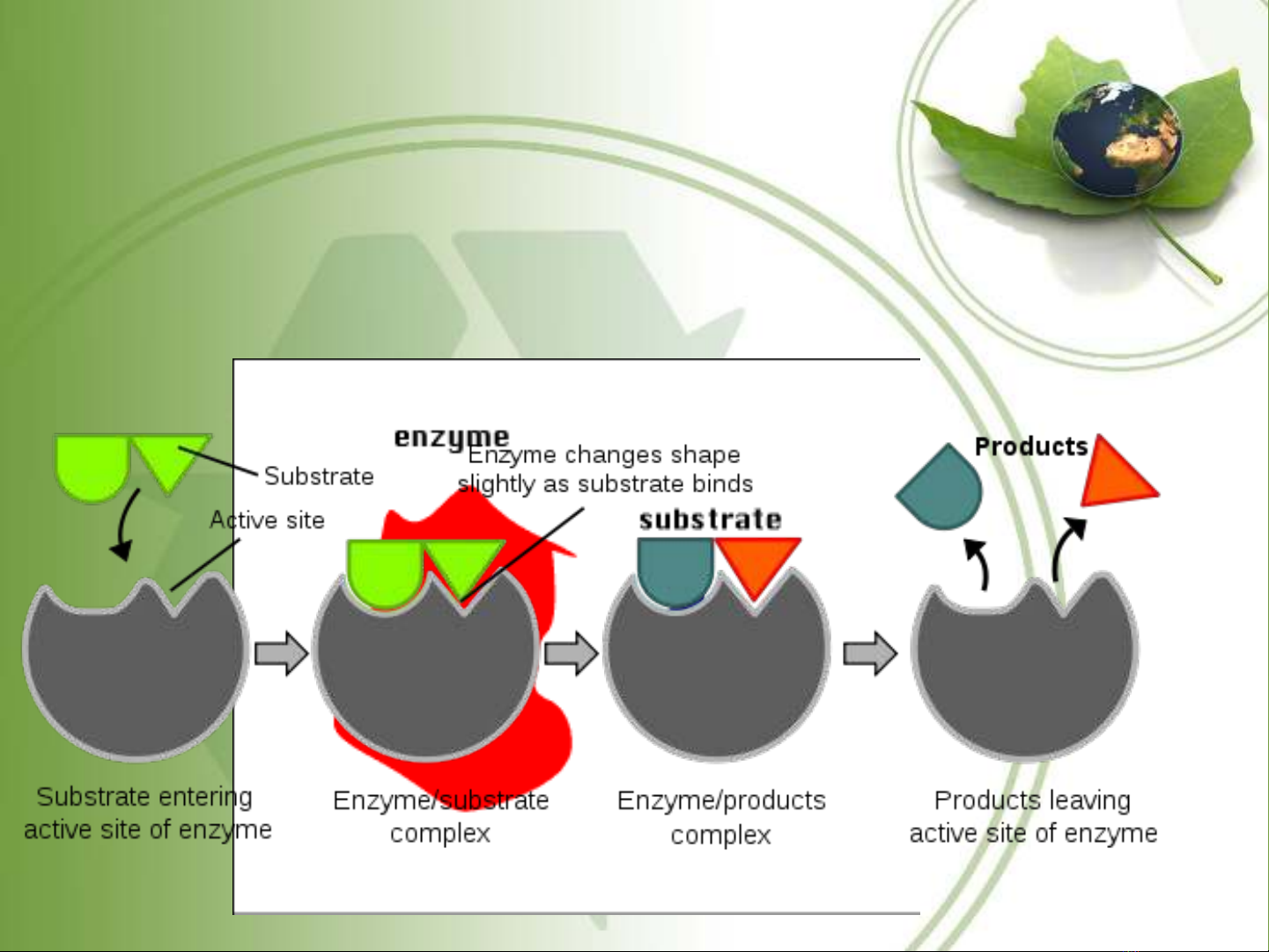
Induced fit theory
•The initial reaction between E and S is weak, but it
rapidly induce by conformational changes in the enzyme.







![Bài giảng Enzyme Công nghiệp [mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2019/20190927/kuronato/135x160/2591569591154.jpg)

![Giáo trình Công nghệ Enzim Phần 2: [Mô tả/Định tính nếu cần]](https://cdn.tailieu.vn/images/document/thumbnail/2016/20160604/tangtuy14/135x160/1762485882.jpg)
![Giáo trình Công nghệ Enzim Phần 1: [Thêm Mô Tả Chi Tiết Hấp Dẫn Hơn Về Nội Dung]](https://cdn.tailieu.vn/images/document/thumbnail/2016/20160604/tangtuy14/135x160/2028746805.jpg)

![Giáo trình Vi sinh vật học môi trường Phần 1: [Thêm thông tin chi tiết nếu có để tối ưu SEO]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20251015/khanhchi0906/135x160/45461768548101.jpg)





![Bài giảng Sinh học đại cương: Sinh thái học [mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250812/oursky02/135x160/99371768295754.jpg)



![Đề cương ôn tập cuối kì môn Sinh học tế bào [Năm học mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2026/20260106/hoang52006/135x160/1251767755234.jpg)

![Cẩm Nang An Toàn Sinh Học Phòng Xét Nghiệm (Ấn Bản 4) [Mới Nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20251225/tangtuy08/135x160/61761766722917.jpg)

