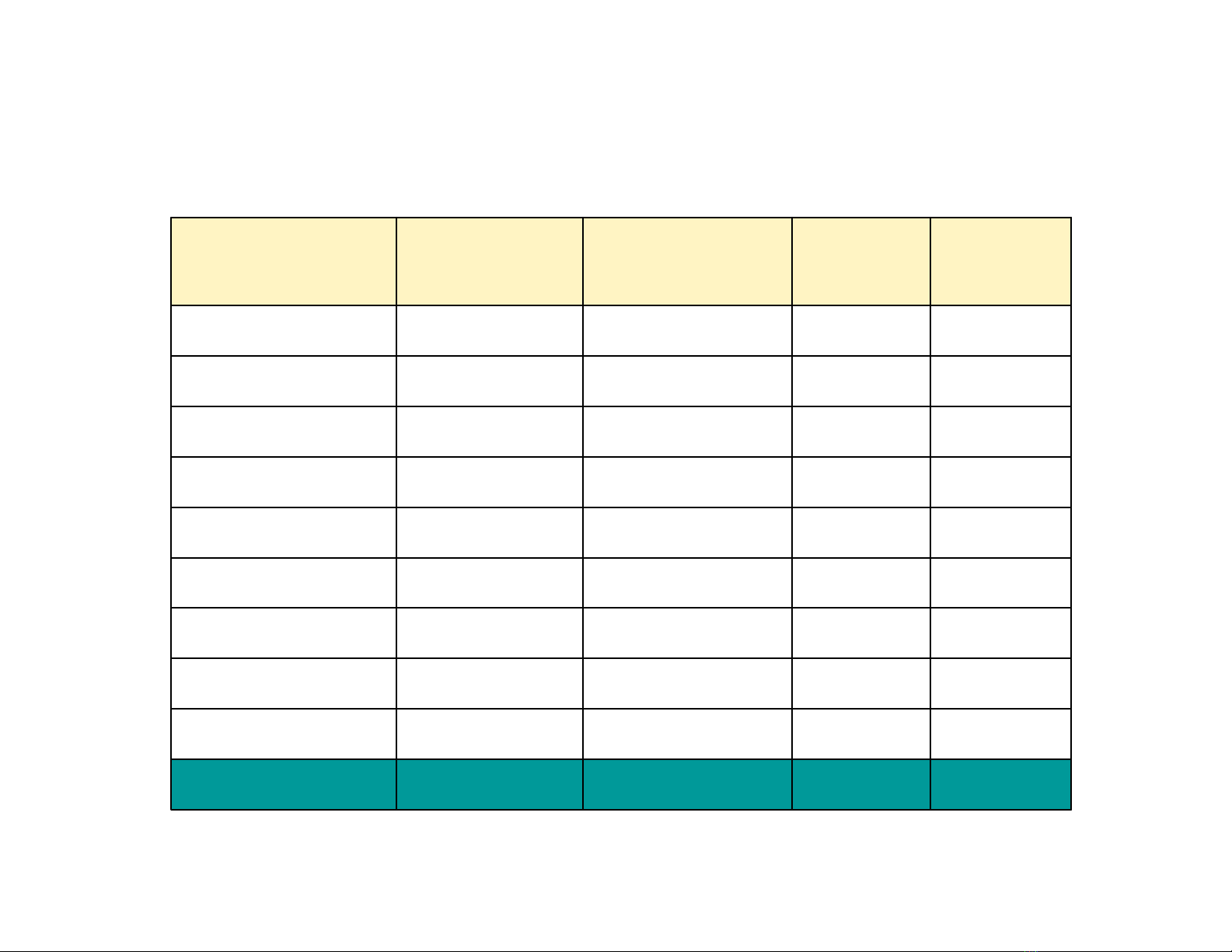
0,0962201,1657062006
0,1768128,940907TB 01 - 05
0,0947153,1442772005
0,16103207,44661832004
0,1769146,91405432003
0,1964376,6219082002
0,2571104,51285842001
0,264969,89187402000
0,286583,6227421999
0,3347455,71239971998
!
"#
$#
"#
%&'()
"#