
Action of palytoxin on apical H
+
/K
+
-ATPase in rat colon
Georgios Scheiner-Bobis
1
, Thomas Hu¨ bschle
2
and Martin Diener
2
1
Institute for Biochemistry and Endocrinology,
2
Institute for Veterinary Physiology, Justus-Liebig-University Giessen, Germany
Palytoxin stimulated a cation-dependent short-circuit cur-
rent (Isc) in rat distal and proximal colon in a concen-
tration-dependent fashion when applied to the mucosal
surface of the tissue. The distal colon exhibited a higher
sensitivity to the toxin. The palytoxin-induced Isc was
blocked by vanadate but was resistant to ouabain or
scilliroside, suggesting the conversion of a vanadate-sen-
sitive H
+
/K
+
-ATPase into an electrogenic cation trans-
porter. Cation substitution experiments with basolaterally
depolarized tissues suggested an apparent permeability of
the palytoxin-induced conductance of Na
+
>K
+
>Li
+
.
Immunohistochemical control experiments confirmed the
absence of the Na
+
/K
+
-ATPase in the apical membrane.
Consequently, the pore-forming action of palytoxin is not
restricted to Na
+
/K
+
-ATPase but is also observed with
the colonic H
+
/K
+
-ATPase.
Keywords: ATPase; colon; palytoxin; Isc; ion channel.
P
2C
-type ATPases are oligomeric enzymes consisting of a
and bsubunits [1]. The sodium pump from the plasma
membranes of animal cells, a member of this group,
generates a sodium gradient by pumping three Na
+
ions
out of the cell and two K
+
ions into the cell for each ATP
hydrolyzed [2]. This sodium gradient is the driving force of
all secondarily active transporters and a presupposition for
neuronal conduction of signals.
The closest relatives of the sodium pump are the proton
pumps from gastric and colon epithelial cells [3]. Although
these pumps are not identical, they both catalyze an active
secretion of protons driven by ATP hydrolysis. Unlike the
sodium pump, however, both proton pumps are electro-
neutral: each transports one K
+
ion from the luminal side
into the cytosol for each H
+
secreted.
Several naturally occurring toxins have been identified as
specific inhibitors of the sodium pump. Among them, the
so-called cardioactive steroids or cardiac glycosides are not
only known for their ability to selectively target the sodium
pump but are widely used as effective medication for
patients with heart failure or heart insufficiency [4]. Paly-
toxin, a toxin isolated from corals of the family Palythoa
(e.g. Palythoa caribaeorum), is also a highly specific inhibitor
of the sodium pump [5,6]. This most potent toxin (for
rodents the LD
50
is 10–250 ng per kg of body weight) of
animal origin can also be found together with ciguatoxin,
maitotoxin, or gambierol in fishes that contribute to
ciguatera poisonings [7,8]. Palytoxin is a rather unique
and large molecule with the structural formula
C
129
H
223
N
3
O
54
. The molecule can be divided into three
subdomains, each connected by peptide bonds: a large
N-terminal polyhydroxy x-amino acid followed by a
dehydro-b-alanine residue and an aminopropanol group.
The number of free hydroxyl groups is 42 [9,10]. Unlike the
cardioactive steroids, however, which inhibit both ATP
hydrolysis and ion conduction, palytoxin acts by arresting
the ionophore of the pump into a permanently open state.
Thus, in this case, inhibition of ATP hydrolysis is no longer
associated with inhibition of ion conductivity.
Yeast cells, which are usually insensitive to palytoxin,
display a palytoxin-induced K
+
efflux when they hetero-
logously express aand bsubunits of the mammalian sodium
pump [11,12]. This flux is sensitive to ouabain (g-strophan-
thine), the most well known inhibitor of the pump. Based on
these and other experiments showing the formation of
palytoxin-induced ion channels in membranes containing
in vitro-translated Na
+
/K
+
-ATPase [13], it is widely
accepted that palytoxin specifically targets the sodium
pump and inhibits its catalytic activity by converting the
ATPase into an ion channel.
Palytoxin action on other P
2C
-type ATPases has not yet
been demonstrated. Thus, in the current investigation, we
describe the action of palytoxin on the H
+
/K
+
-ATPase
from the rat colon and demonstrate that the interaction of
this toxin with the enzyme results in specific currents that
are similar to those observed from its action on sodium
pumps.
EXPERIMENTAL PROCEDURES
Solutions
The Ussing chamber experiments were carried out in a
bathing solution containing according to Parsons &
Paterson [14] (mmolÆL
)1
): NaCl, 107; KCl, 4.5; NaHCO
3
,
25; Na
2
HPO
4
,1.8;NaH
2
PO
4
,0.2;CaCl
2
,1.25;MgSO
4
,1;
andglucose,12.Thesolutionwasgassedwithamixtureof
5% CO
2
and 95% O
2
; the pH was 7.4. For depolarization of
the basolateral membrane, a modified bathing solution was
used in which NaCl was replaced by 111.5 mmolÆL
)1
KCl.
In the LiCl bathing solution, NaCl was replaced equimo-
larly by LiCl.
Correspondence to G. Scheiner-Bobis, Institut fu
¨r Biochemie und
Endokrinologie, Justus-Liebig-Universita
¨t Gießen, Frankfurter Str.
100, D-35392 Gießen, Germany.
Fax: + 49 641 99 38189, Tel.: + 49 641 99 38180,
E-mail: Georgios.Scheiner-Bobis@vetmed.uni-giessen.de
Abbreviations:NMDG,N-methyl-
D
-glucamine; Gt, tissue
conductance; Isc, short-circuit current.
(Received 11 March 2002, revised 14 May 2002,
accepted 18 June 2002)
Eur. J. Biochem. 269, 3905–3911 (2002) FEBS 2002 doi:10.1046/j.1432-1033.2002.03056.x

Tissue preparation
Wistar rats were used with a weight of 180–220 g. The
animals had free access to water and food until the day of
the experiment. Animals were stunned by a blow on the
head and killed by exsanguination (approved by Regi-
erungspra
¨sidium Giessen, Giessen, Germany). The serosa
and muscularis propria were stripped away by hand to
obtain the mucosa-submucosa preparation of the distal part
of the colon descendens. Two distal and two proximal
segments of the colon of each rat were prepared.
Short-circuit current measurement
The tissue was mounted in a modified Ussing chamber,
bathed with a volume of 3.5 mL (see above) on each side of
the mucosa and short-circuited by a voltage clamp (Ing.
Buero Mussler, Aachen, Germany) with correction for
solution resistance as described previously [15]. The exposed
surface of the tissue was 1 cm
2
. Short-circuit current (Isc)
was continuously recorded and tissue conductance (Gt) was
measured every min. Isc is expressed as lAÆh
)1
Æcm
)2
, i.e. the
flux of a monovalent ion per time and area with
1lEqÆh
)1
Æcm
)2
¼26.9 lAÆcm
)2
. Tissues were left for 1 h
to stabilize the Isc before the effect of drugs was studied. The
baseline electrical parameters were determined as the mean
obtained during 3 min just before administration of a drug.
Immunohistochemical detection of the Na
+
/K
+
-ATPase
in colonic epithelium
Wistar rats (n¼2) were anesthetized with sodium pento-
barbital (60 mgÆkg
)1
body weight; Narcoren, Merial
GmbH, Hallbergmoos, Germany) and transcardially per-
fused with 4% paraformaldehyde in 100 mmolÆL
)1
phos-
phate buffer (pH 7.2). The distal colon was removed and
postfixed in the same fixative for 1 h at room temperature
and then the tissue was cryoprotected in 20% sucrose in
phosphate buffer overnight at 4 C. Tissue was cut the
following day.
Coronal 10–12 lm colonic sections were cut on a cryostat
(model HM 500, Microm, Walldorf, Germany). To detect
Na
+
/K
+
-ATPase immunoreactivity, a commercial tyra-
mide amplification kit (NEL700, NEN Life Science Prod-
ucts GmbH, Cologne, Germany), based on the catalyzed
reporter deposition method, was used. Tyramide amplifica-
tion staining was performed according to the kit description
in a phosphate buffer system (pH 7.2). In detail, sections
were placed in 10% fetal bovine serum containing 0.3%
Triton X-100 for 1 h at room temperature. Incubation with
the primary anti-(Na
+
/K
+
-ATPase) Ig (MA3-929, mon-
oclonal, mouse, a
1
-subunit, Affinity BioReagents, Golden,
CO, USA) was performed for 24–36 h at 4 C at a dilution
of 1 : 150 to 1 : 5000). The primary antibody was then
detected with a secondary biotinylated anti-(mouse IgG) Ig
(1 : 200, Vector BA-2001, Linaris Biologische Produkte,
Wertheim-Bettingen, Germany) for 1 h at room tempera-
ture. After amplification, the immunohistochemical
processing was finished with 1 : 200 fluorescein (FITC)-
conjugated avidin D (Vector, Linaris Biologische Produkte,
Wertheim-Bettingen, Germany). In order to demonstrate
the overall morphology of the colonic epithelium, parallel
series of sections adjacent to cryosections of the immuno-
fluorescent-stained series were cut for light-microscopic
analysis and consequently counterstained using cresylviolet.
Finally, these sections were cover slipped with Entellan
(Merck, Darmstadt, Germany) while immunofluorescent
sections were cover slipped with crystal/mount (Biomedia,
FosterCity, USA).
Microscopic analysis
Sections were analyzed using a an Olympus BX50 light/
fluorescent microscope (Olympus Optical Co., Hamburg,
Germany). For light microscopy, digital images were
taken with an Olympus Camedia 3030 camera using the
Olympus
CAMEDIA MASTER
software package (Olympus
Optical Co., Hamburg, Germany). For fluorescent
microscopy, digital images were taken with a Visicam
(PCO Computer Optics, Kehlheim, Germany) using the
METAMORPH/METAFLUOR
software package (Visitron
Systems, Puchheim, Germany). Image editing software
(
ADOBE PHOTOSHOP
) was used to adjust brightness and
contrast and to combine the individual images into the
greyscale mode figure plate.
Drugs
Palytoxin (purchased from L. Be
´ress, Institute for Toxicol-
ogy, University of Kiel, Germany) was dissolved in
10 mmolÆL
)1
Hepes, 0.5 mmolÆL
)1
Tris, 1 mmolÆL
)1
CaCl
2
and 1 gÆL
)1
BSA. Sodium orthovanadate (Calbiochem, Bad
Soden, Germany) was dissolved in an aqueous stock
solution. Ouabain was dissolved in dimethylsulfoxide (final
concentration 2.5 lLÆmL
)1
), scilliroside (Sandoz, Basel,
Switzerland) was dissolved in methanol (final concentration
2.5 lLÆmL
)1
). If not indicated differently, drugs were from
Sigma, Deisenhofen, Germany.
Statistics
Results are given as means ± SEM. When the means of
several groups were compared, an analysis of variances was
first performed. If the analysis of variances indicated
significant differences between the groups investigated,
further comparison was carried out by a Student’s t-test
(paired or unpaired as appropriate) or by the Mann–
Whitney U-test. An F-test was applied to decide which test
method was to be used.
RESULTS
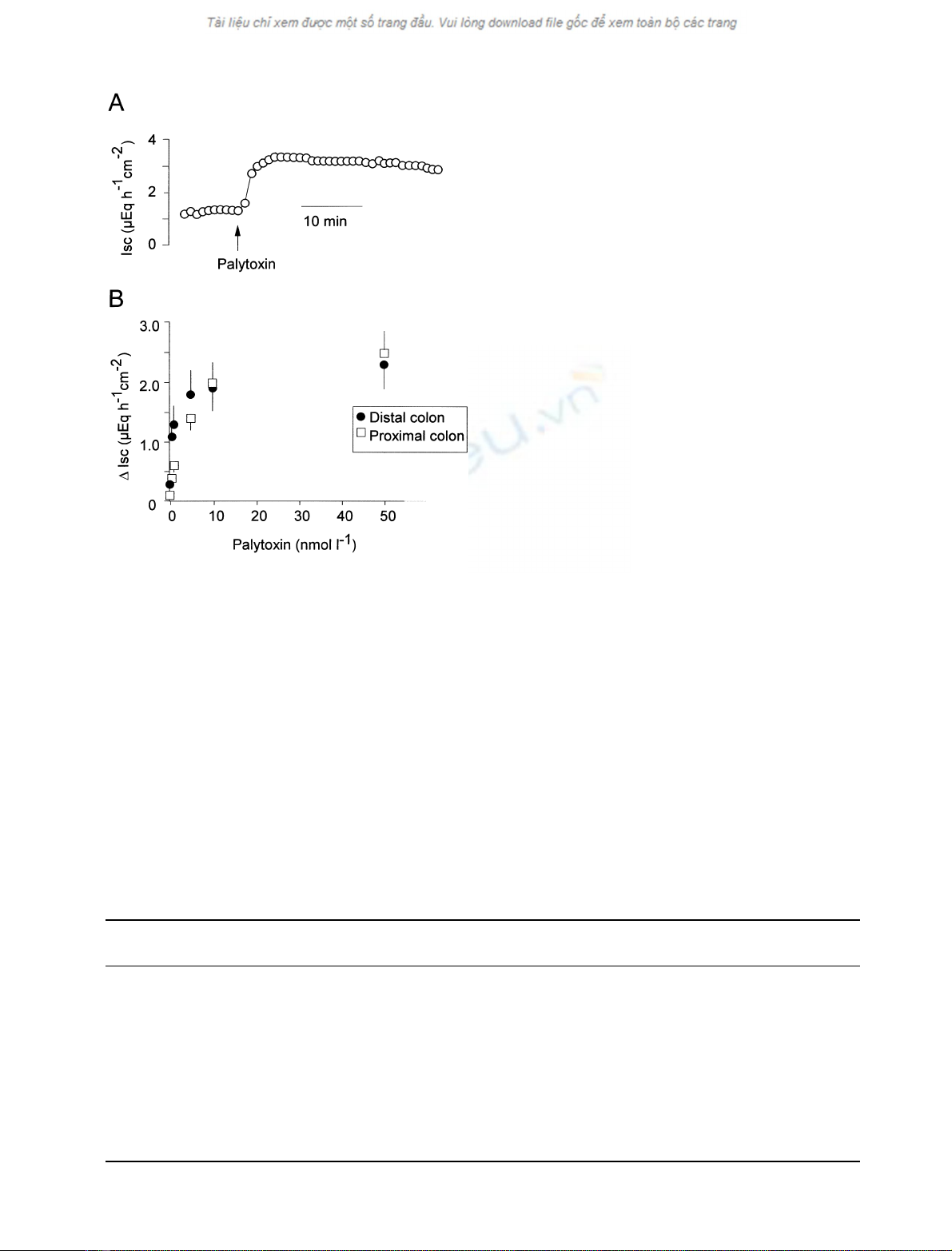
Basal effects of palytoxin
Palytoxin (10
)8
molÆL
)1
on the mucosal side) induced an
increase in short-circuit current (Isc) in rat distal and
proximal colon (Fig. 1A). The response started immediately
after administration of the toxin and was stable at least for
30 min The effect was concentration-dependent (Fig. 1B).
A first, significant increase in Isc occurred at a concentration
of 10
)10
molÆL
)1
. Similar effects were observed in the distal
and proximal colon, although the potency of palytoxin
appeared to be higher in the distal than in the proximal
colon. The increase in Isc was concomitant with a rise in
tissue conductance (Gt). At the highest concentration of
palytoxin tested (5 ·10
)8
molÆL
)1
), Gt increased by
3906 G. Scheiner-Bobis et al. (Eur. J. Biochem. 269)FEBS 2002
7.1 ± 1.7 msÆcm
)2
in the distal and by 8.9 ± 2.7 msÆcm
)2
in the proximal colon (n¼6–8, p< 0.05 for both colonic
segments).
The effect of palytoxin was enhanced in the presence of
mucosal borate (0.5 mmolÆL
)1
), especially in the proximal
colon (Table 1). Similar observations were made in the past
concerning palytoxin effects on erythrocytes, neurosyna-
ptosomes or yeast cells that express mammalian sodium
pumps [6,11]. Although no real evidence exists about the
role of borate, borate alone does not induce any cation
fluxes from erythrocytes [6] or from yeast expressing the
mammalian sodium pump [11] or from the colon tissues
investigated here. It is possible that borate interacts with
some of the 42 free hydroxyl groups of palytoxin, similarly
to the way it interacts with carbohydrates. It also might be
that it interacts with the carbohydrates of the strongly
glycosylated bsubunits of the P
2C
-type ATPases. These
possible complexes might induce a particular conformation
of the palytoxin molecule or of the enzyme that favors
mutual interaction between the two reactants. Therefore, all
subsequent experiments were carried out with borate in the
mucosal solution using a palytoxin concentration of
10
)8
molÆL
)1
.
For theoretical reasons it is not possible that admin-
istration of palytoxin to the basolateral side of an
epithelium can induce an Isc. If the toxin converts the
Na
+
/K
+
-pump into a cation channel, the cytosolic Na
+
concentration will increase and finally reach the extracel-
lular concentration, whereas the cytosolic K
+
concentra-
tion will fall to the level at the extracellular side. Thus
there is no more driving force for any active ion
movement, i.e. there will be no short-circuit current
response. Therefore, as expected, when applied at the
serosal side, in six independent experiments the toxin had
no effect on Isc (data not shown).
Sensitivity against inhibitors of ATPases
The effect of palytoxin (10
)8
molÆL
)1
at the mucosal side)
was resistant to mucosal ouabain (10
)3
molÆL
)1
) or scill-
iroside (10
)4
molÆL
)1
at the mucosal side) (Table 1), a
potent blocker of the Na
+
/K
+
-pumpinrattissue[16].All
pump inhibitors were administered 1 h prior to palytoxin;
for effects of the blockers on baseline Isc, see Table 2. In
contrast, pretreatment with sodium orthovanadate
(10
)4
molÆL
)1
at the mucosal side) nearly suppressed the
action of palytoxin (Table 1).
Table 1. Effect of palytoxin on Isc under different conditions. The increase in Isc evoked by palytoxin (10
)8
molÆL
)1
at the mucosal side) was
measured in the absence of any drugs or in the presence of Tris borate (10
)4
molÆL
)1
at the mucosal side; pretreated for 15 min), ouabain
(10
)3
molÆL
)1
at the mucosal side; pretreated for 1 h), scilliroside (10
)4
molÆL
)1
at the mucosal side; pretreated for 1 h), or vanadate (10
)4
molÆL
)1
at the mucosal side; pretreated for 1 h), or after replacement of NaCl by NMDG chloride (107 mmolÆL
)1
NMDG chloride buffer at the mucosal
side). *p< 0.05 vs. baseline, p< 0.05 vs. response to palytoxin in the absence of any drugs.
Distal colon
DIsc (lEqÆh
)1
Æcm
)2
)
Proximal colon
DIsc (lEqÆh
)1
Æcm
)2
)n
Palytoxin 2.7 ± 0.6* 0.3 ± 0.2 5–8
Palytoxin + borate 4.2 ± 0.7* 1.9 ± 0.4* 5–9
Palytoxin 2.8 ± 0.8* 2.0 ± 0.9 5–9
Palytoxin + ouabain 2.3 ± 0.7* 2.2 ± 0.6* 6–9
Palytoxin + vanadate 0.4 ± 0.1* 0.3 ± 0.1 6
Palytoxin + scilliroside 2.1 ± 0.6* 1.1 ± 0.5 6–8
Palytoxin, Na ± free 0.1 ± 0.1 0.1 ± 0.1 6
Basolateral depolarization 0.5 ± 0.1* 0.1 ± 0.1 5–6
(apical Na
+
)
Basolateral depolarization )1.7 ± 0.5* )0.9 ± 0.2* 6–8
(apical Li
+
)
Fig. 1. Induction of a short-circuit current in rat distal and proximal
colon by palytoxin. (A) Typical Isc response evoked by palytoxin
(10
)8
molÆL
)1
at the mucosal side in the presence of 0.5 mmolÆL
)1
Na
borate at the mucosal side). (B) Concentration-dependent increase in
Iscabovebaseline(DIsc) evoked by palytoxin in the distal (closed
circles) and proximal (open rectangles) rat colon. Palytoxin was
administered cumulatively at the mucosal side in the presence of
0.5 mmolÆL
)1
Na borate. Values are means ± SEM, n¼6–8.
FEBS 2002 Palytoxin action on colonic H
+
/K
+
-ATPase (Eur. J. Biochem. 269) 3907


























