
Soluble guanylate cyclase is allosterically inhibited by direct
interaction with 2-substituted adenine nucleotides
Inez Ruiz-Stewart, Shiva Kazerounian, Giovanni M. Pitari, Stephanie Schulz and Scott A. Waldman
Division of Clinical Pharmacology, Departments of Medicine and Biochemistry and Molecular Pharmacology, Thomas Jefferson
University, Philadelphia, PA, USA
Nitric oxide (NO), the principal endogenous ligand for sol-
uble guanylate cyclase (sGC), stimulates that enzyme and
accumulation of intracellular cGMP, which mediates many
of the (patho) physiological effects of NO. Previous studies
demonstrated that 2-substituted adenine nucleotides, inclu-
ding 2-methylthioATP (2MeSATP) and 2-chloroATP
(2ClATP), allosterically inhibit guanylate cyclase C, the
membrane-bound receptor for the Escherichia coli heat-
stable enterotoxin in the intestine. The present study exam-
ined the effects of 2-substituted adenine nucleotides on crude
and purified sGC. 2-Substituted nucleotides inhibited basal
and NO-activated crude and purified sGC, when Mg
2+
served as the substrate cation cofactor. Similarly, 2-substi-
tuted adenine nucleotides inhibited those enzymes when
Mn
2+
, which activates sGC in a ligand-independent fashion,
served as the substrate cation cofactor. Inhibition of sGC
by 2-substituted nucleotides was associated with a decrease
in V
max
, consistent with a noncompetitive mechanism. In
contrast to guanylate cyclase C, 2-substituted nucleotides
inhibited sGC by a guanine nucleotide-independent mech-
anism. These studies demonstrate that 2-substituted adenine
nucleotides allosterically inhibit basal and ligand-stimulated
sGC. They support the suggestion that allosteric inhibition
by adenine nucleotides is a general characteristic of the
family of guanylate cyclases. This allosteric inhibition is
mediated by direct interaction of adenine nucleotides with
sGC, likely at the catalytic domain in a region outside the
substrate-binding site.
Keywords: soluble guanylate cyclase; adenine nucleotide.
Cyclic GMP (cGMP) is an important signaling molecule
that regulates many physiological functions, including
vascular smooth muscle motility, intestinal fluid and
electrolyte homeostasis, cellular proliferation, and photo-
transduction (reviewed in [1]). The family of enzymes that
synthesize cGMP from GTP, the guanylate cyclases, are
expressed by most tissues in the cytoplasmic (soluble) and
membrane (particulate) compartments [2–4]. These enzymes
can be activated by specific ligands or by free Mn
2+
through
ligand-independent mechanisms, and require a divalent
cation (Mn
2+
or Mg
2+
) as an essential cofactor for catalytic
activity [5].
Particulate guanylate cyclases (pGCs) are multidomain
homo-oligomers and each monomer contains an extracellu-
lar ligand-binding domain, a single transmembrane domain,
an intracellular kinase homology domain (KHD) and a
catalytic domain (reviewed in [1]). Soluble guanylate cyclases
(sGCs) are heterodimers composed of aand bsubunits and
each monomer contains a heme binding domain, a dimeri-
zation domain, and a catalytic domain [1,6]. The primary
structure of the catalytic domains of sGC and pGC are
homologous, reflecting their similarity of function [7,8].
pGCs are allosterically regulated by adenine nucleotides in
a complex fashion. When Mg
2+
serves as the cation cofactor,
ATP potentiates ligand activation of pGCs presumably by
binding to the KHD. The working hypothesis suggests that
the KHD is intrinsically inhibitory and ligand–receptor
interaction permits association of that domain with ATP
resulting in derepression of the catalytic domain [9–11]. It
remains unclear whether ATP binding to the KHD dere-
presses the enzyme or an intrinsic kinase activity mediates
derepression [12]. In addition, ligand activation of pGCs is
dependent upon the phosphorylation state of serine and
threonine residues within the KHD, which, in turn, is
dependent upon ATP [13,14]. Indeed, one mechanism by
which desensitization of pGCs may be mediated is ligand-
dependent dephosphorylation of those residues [15–17].
Recently, a novel allosteric mechanism mediating inhibi-
tion of pGC by adenine nucleotides was identified. Thus,
adenine nucleotides substituted in the 2-position of the
purine ring inhibited the isoform of pGC expressed in
intestinal epithelial cells, GC-C, the receptor for ST that is a
major cause of diarrhea in animals and humans [18]. Indeed,
2ClATP and 2MeSATP inhibited basal and ST-stimulated
GC-C in a concentration-dependent manner with a K
i
10
)4
M
[19]. Allosteric inhibition by those nucleotides was
associated with a decrease in V
max
, characteristic of a
noncompetitive mechanism and was mediated by the
intracellular domains of GC-C [19]. Furthermore, inhibition
Correspondence to I. Ruiz-Stewart, Division of Clinical Pharmacology,
Thomas Jefferson University, 1100 Walnut Street, MOB 810,
Philadelphia, PA 19107, USA. Fax: +1 215 955 7006,
Tel.: +1 215 955 0054,
E-mail: iar001@jefferson.edu
Abbreviations: cGMP, cyclic GMP; 2ClAdo, 2-chloroadenosine;
2ClATP, 2-chloroadenosine triphosphate; GCA, guanylate cyclase A;
GC-C, guanylate cyclase C; GTPcS, guanosine 5¢O-(3-triphosphate);
IBMX, isobutylmethylxanthine; KHD, kinase homology domain
2MeSATP, 2-methylthioadenosine triphosphate; NO, nitric oxide;
pGC, particulate guanylate cyclase; sGC, soluble guanylate cyclase;
SNP, sodium nitroprusside; ST, Escherichia coli heat-stable entero-
toxin.
(Received 3 December 2001, revised 4 March 2002,
accepted 11 March 2002)
Eur. J. Biochem. 269, 2186–2193 (2002) FEBS 2002 doi:10.1046/j.1432-1033.2002.02874.x
of GC-C by 2-substituted nucleotides was guanine nucleo-
tide-dependent, suggesting a role for a guanine nucleotide-
binding protein in the mechanism mediating allosteric
inhibition of GC-C [19]. Incubation of intestinal epithelial
cells in vitro with 2ClAdo, which undergoes intracellular
transformation to 2ClATP, prevented ST-induced [cGMP]
i
accumulation and electrolyte transport [20].
While ligand activation by pGCs is regulated in a
complex fashion by adenine nucleotides, there appears to
be a less well-defined role for those nucleotides in the
regulation of NO-activation of sGC. ATP does not activate
basal sGC nor is it required to potentiate activation of sGC
by NO. Indeed, sGC lacks the KHD present in all known
mammalian pGCs that presumably mediates allosteric
activation of those enzymes. Previous studies have demon-
strated that phosphorylation of sGC by cAMP-dependent
protein kinase and protein kinase C increases the respon-
siveness of that enzyme to NO [21,22]. Although adenine
nucleotides do not appear to be absolutely required for
ligand activation, their ability to allosterically inhibit sGC
remains unclear. In this study, we examine the allosteric
regulation of crude and purified basal, and NO-activated
sGC by 2-substituted adenine nucleotides.
MATERIALS AND METHODS
Cell culture
T84 cells (ATCC, Rockville, MD, USA) were grown
at 37 C in Dulbecco’s modified Eagle’s medium/F12
(Mediatech, Herndon, VA, USA), 10% fetal bovine serum
(Mediatech, Herndon, VA, USA), and 1% penicillin/
streptomycin (Gibco, Grand Island, NY, USA) in a humi-
dified atmosphere of 5% CO
2
[23].
Preparation of membranes
Confluent cells were washed twice with TED [50 m
M
Tris/
HCL (pH 7.5) containing 1 m
M
EDTA, 1 m
M
dithothre-
itol, and 1 m
M
phenylmethanesulfoxide], collected by
scraping into 5 mL of TED, and homogenized on ice in
TED using a Wheaton overhead stirrer. Homogenates were
centrifuged (4 C) at 100 000 gfor 60 min to produce a
pellet, which was then resuspended in TED at 2 mg
proteinÆmL
)1
. Membranes were stored at )20 Cand
frozen-thawed once only for analyses.
Preparation of crude sGC
Rat lungs (Pelfreeze, Rogers, AR, USA) were washed in ice-
cold 0.9% NaCl to remove residual blood. Lungs were
homogenized on ice with a Wheaton overhead stirrer in
9 vol.(w/v)ofTEDS(20 m
M
Tris/HCl (pH 7.5) containing,
1m
M
EDTA, 1 m
M
dithiothreitol, and 250 m
M
sucrose)
followed by centrifugation (4 C) at 100 000 gfor 60 min.
Supernatants were recovered, adjusted to 2 mg pro-
teinÆmL
)1
with TEDS, stored at )20 C and frozen-thawed
once only for analyses.
Guanylate cyclase activity
Guanylate cyclase activity was quantified as described
previously [24]. Briefly, 20 lg of supernatant or membrane
protein were incubated for 5 min at 37 Cin100lLof
50 m
M
Tris buffer (pH 7.5), which contained 500 l
M
isobutylmethylxanthine (IBMX), 15 m
M
creatine phos-
phate, 2.7 U of creatine phosphokinase, MgCl
2
or MnCl
2
(3 m
M
in excess of nucleotide), and GTP, activating ligand,
and 2-substituted adenine nucleotide as indicated in the
figure legend. For sGC purified from bovine lung (Alexis
Biochemical Corporation, San Diego, CA, USA), 5 ng of
protein was incubated for 5 min at 37 C in 100 lLof
50 m
M
Tris buffer (pH 7.4), 0.5 mgÆmL
)1
BSA, 1 m
M
dithiothreitol, MgCl
2
or MnCl
2
(3 m
M
in excess of nucleo-
tide unless otherwise stated), and GTP, 50 l
M
SNP, and 2-
substituted adenine nucleotides where indicated. Enzyme
reactions were terminated by the addition of 50 m
M
sodium
acetate (pH 4.0) followed by boiling for 3 min. Samples
were acetylated and cGMP production quantified by
radioimmunoassay [20]. All enzyme reactions were per-
formed in duplicate and radioimmunoassays were per-
formed in triplicate. Results reflect enzyme activities that
were linear with respect to time and protein concentrations.
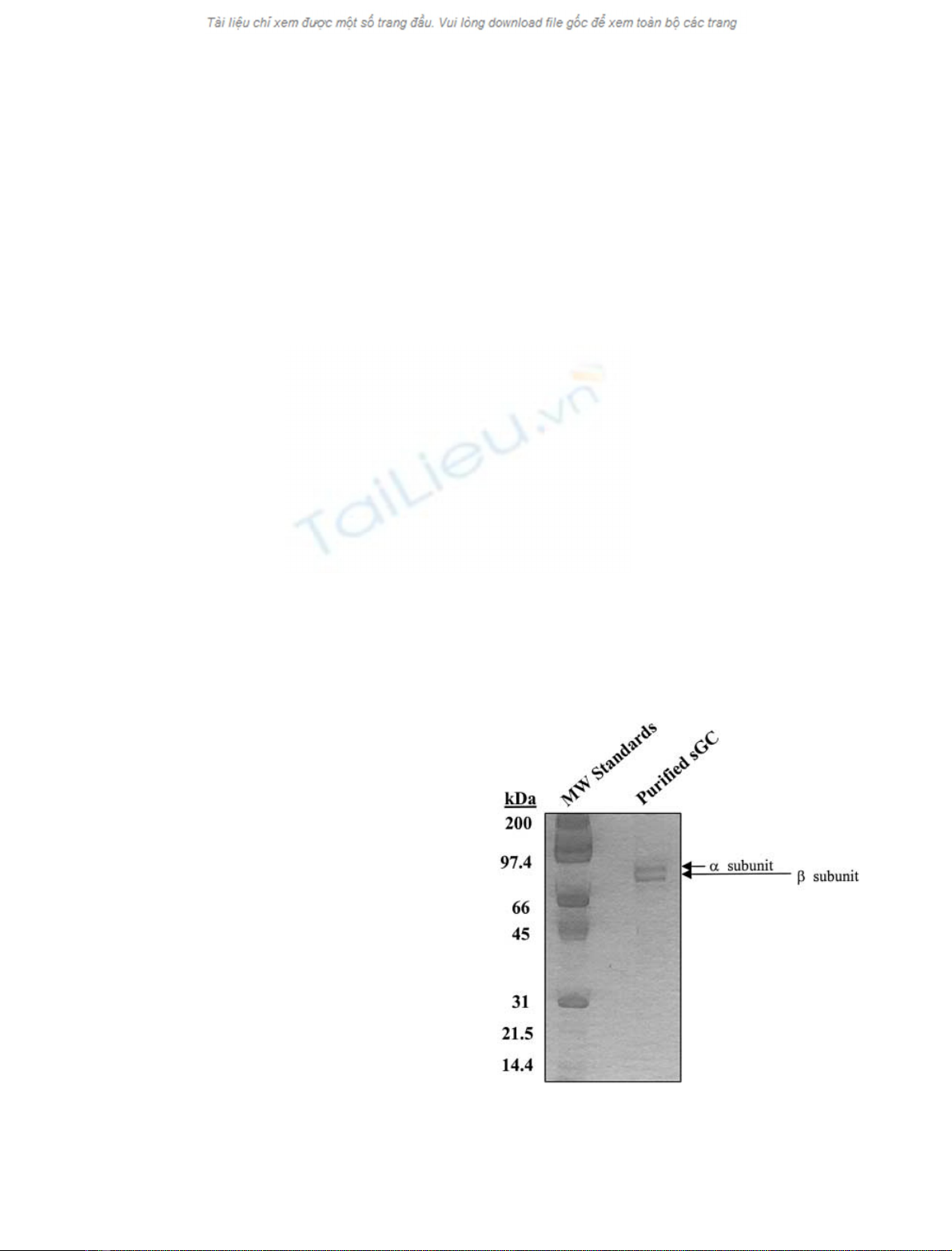
Purified sGC
sGC (1.25 lg), purified by immunoaffinity chromatography
employing an antibody to the C-terminus of the b1 subunit
[25], was analyzed by SDS/PAGE on a precast 8 ·10 cm
12.5% polyacrylamide gel (Owl, Portsmouth, NH, USA) as
described previously [25]. The gel, stained with Gelcode Blue
(Pierce, Rockford, IL, USA), demonstrated that these
preparations were composed of 73- and 70-kDa proteins
(the aand bsubunits, respectively) (Fig. 1). Densitometric
analysis of these preparations following SDS/PAGE
revealed that > 95% of their composition was aand
bsubunits (data not shown). These observations are iden-
Fig. 1. SDS/PAGE analysis of sGC immunopurified from bovine lung.
sGC (1.25 lg) immunopurified from bovine lung was subjected to
SDS/PAGE on a 12.5% polyacrylamide gel and stained with Gelcode
Blue, as described in Materials and methods.
FEBS 2002 Adenine nucleotides directly inhibit sGC (Eur. J. Biochem. 269) 2187

tical to those reported previously for purification of this
enzyme by immunoaffinity chromatography employing the
same antibody [25].
Miscellaneous
All results are representative of three experiments. 2-subti-
tuted adenine nucleotides, EDTA, dithiothreitol, phenyl-
methanesulfoxide, sodium nitroprusside (SNP), GTP,
IBMX, creatine phosphate, and creatine phosphokinase
were obtained from Sigma (St Louis, MO, USA). Protein
concentration was determined according to the Bradford
method (Bio-Rad, Hercules, CA, USA). Statistical signifi-
cance was analyzed employing Student’s t-test.
RESULTS
Previous studies demonstrated that 1 m
M
2MeSATP or
2ClATP inhibited basal and ST- and Mn
2+
-activated GC-C
(Fig. 2A) [19,20,26]. Similarly, 1 m
M
2MeSATP or 2ClATP
inhibitedbasalandNO-andMn
2+
-stimulated crude rat
lung sGC (Fig. 2B). These nucleotides inhibited basal sGC
60%, NO-activated enzyme 50%, and Mn
2+
-activated
sGC 90%. In addition, 1 m
M
2MeSATP or 2ClATP
inhibited basal and NO- and Mn
2+
-stimulated sGC puri-
fied to apparent homogeneity (Figs 1 and 2C). Inhibition of
crude and purified sGC was comparable suggesting that
factors important for mediating 2-substituted adenine
nucleotide inhibition were not removed during immunopu-
rification. This is the first demonstration that 2-substituted
nucleotides inhibit guanylate cyclase by directly interacting
with the purified enzyme, without a requirement for an
intermediate cofactor [26].
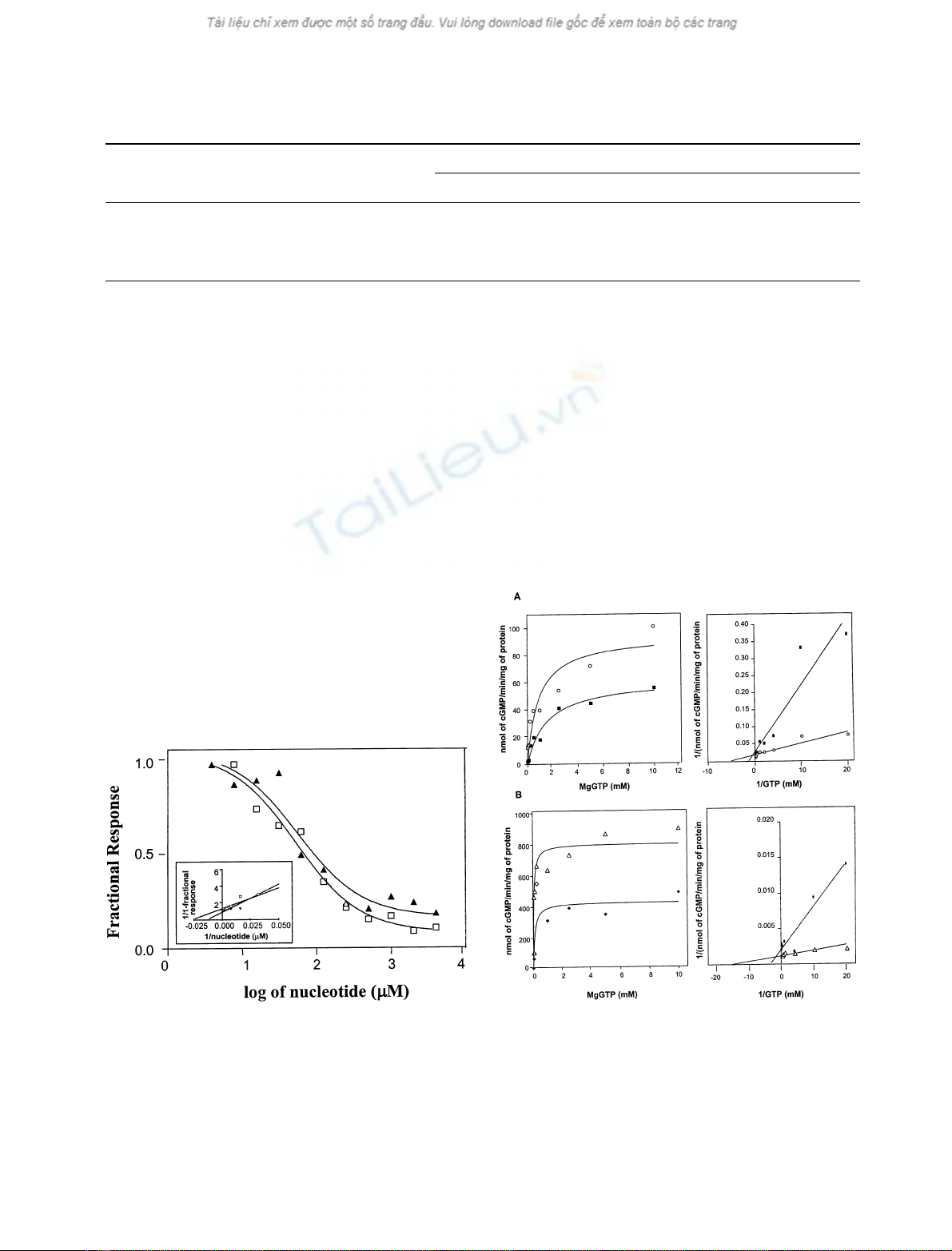
2MeSATP and 2ClATP inhibited basal and NO-activa-
ted crude and purified sGC in a concentration-dependent
and saturable fashion (Fig. 3). These preparations were
maximally inhibited ‡80% by those nucleotides. The K
i
for
inhibition of sGC by those nucleotides was 10
)4
M
and
there were no significant differences in their potency
(Table 1). The potencies of adenine nucleotides to inhibit
crude and purified sGC (K
i
; Table 1) are comparable to
those reported for inhibition of GC-C [19]. That the
pharmacological characteristics of inhibition by 2-substi-
tuted nucleotides were virtually identical for crude and
purified sGC supports the suggestion that this inhibition is
mediated by direct interaction of those nucleotides with
sGC.
Mn
2+
activates sGC and pGCs in a ligand-independent
fashion [1,2,5]. 2MeSATP and 2ClATP inhibited GC-C
activity when either Mn
2+
or Mg
2+
was employed as the
substrate cation cofactor [19,20,26]. Similarly, those nucleo-
tides maximally inhibited purified sGC activity > 80% in a
Fig. 2. Effect of 2-substituted adenine nucleotides on GC-C and sGC.
GC-C and sGC activities were determined as described in Materials
and methods. Incubations contained 1 l
M
ST, 50 l
M
SNP, 3 m
M
excess Mg
2+
or Mn
2+
,or1m
M
2ClATP or 2MeSATP, where indi-
cated. (A) GC-C in T84 cell membranes; (B) crude sGC extracted from
rat lung; (C) sGC purified from bovine lung.
Fig. 3. Concentration-dependence of inhibition of crude and purified
sGC by 2-substituted adenine nucleotides employing Mg
2+
as the sub-
strate cation cofactor. Guanylate cyclase activity was measured in the
presence of varying concentrations of 2MeSATP (h)or2ClATP(m)
in the absence (upper panels) or presence (lower panels) of 50 l
M
SNP.
Enzyme activities are expressed as the ratio of [(enzyme activity in the
presence of nucleotide)/(enzyme activity in the absence of nucleotide)]
(fractional response). Basal activities of crude and purified sGC
were 13.7±1.2pmol cGMPmin
)1
Æmg
)1
of protein and 77.3 ±
33.03 nmol cGMP min
)1
Æmg
)1
of protein, respectively. Activities of
crude and purified sGC stimulated by SNP were 100 ± 18 pmol
cGMP min
)1
Æmg
)1
of protein and 1.8 ± 0.5 lmol of cGMP per
minÆmg
)1
of protein, respectively. Nonlinear regression analysis of the
sigmoidial plots for each of the nucleotides was used to estimate the K
i
values presented in Table 1.
2188 I. Ruiz-Stewart et al. (Eur. J. Biochem. 269)FEBS 2002
concentration-dependent fashion when Mn
2+
was utilized
as the substrate cofactor (Fig. 4). Interestingly, the potencies
of 2-substituted nucleotides to inhibit sGC significantly
increased employing Mn
2+
as the cation cofactor. Thus, the
K
i
values of 2MeSATP and 2ClATP decreased greater
than ninefold in the presence of Mn
2+
compared to Mg
2+
(Table 1)
.
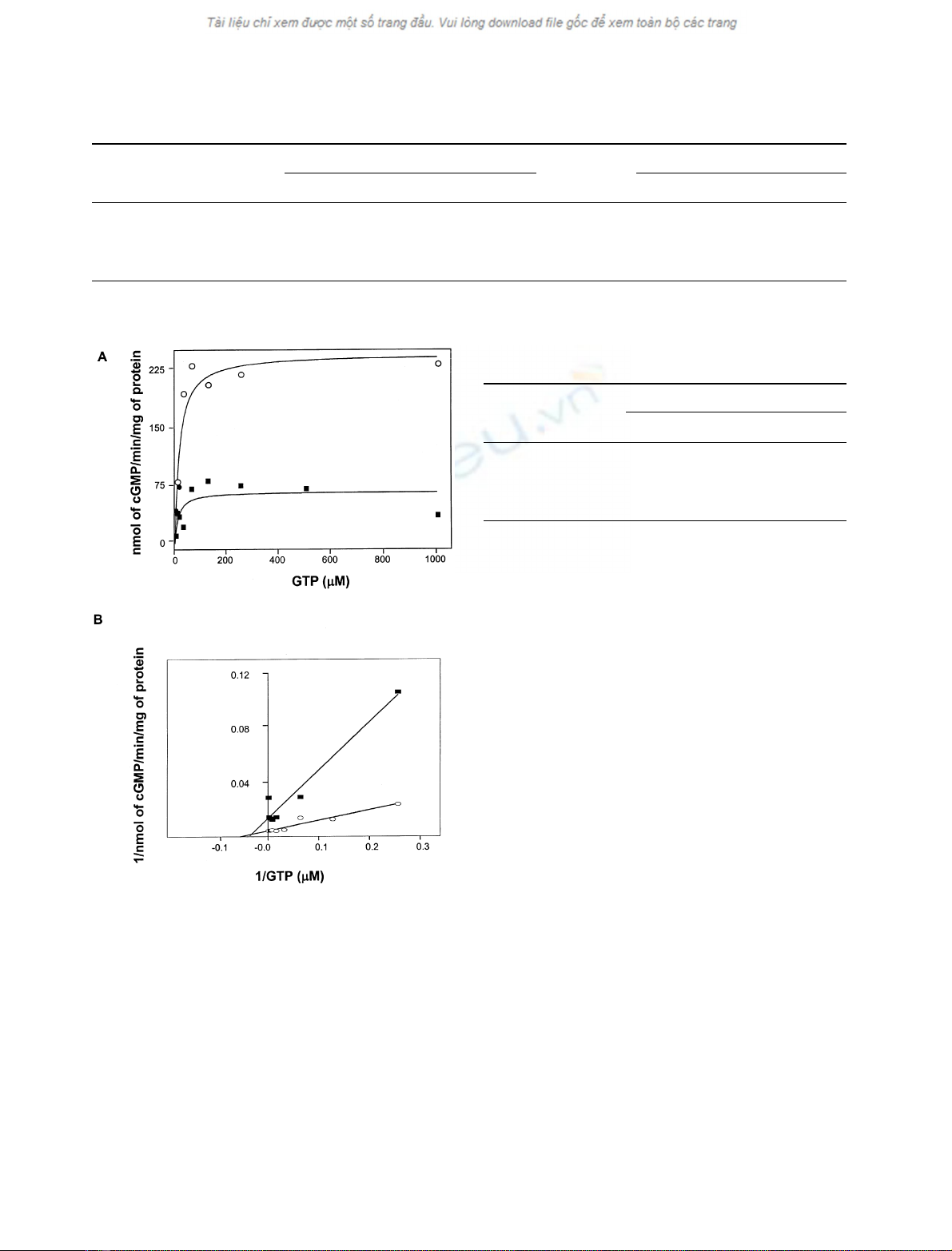
The effects of 2-substituted nucleotides on sGC activity
were examined in the presence of increasing concentrations
of substrate. Employing Mg
2+
as the substrate cofactor,
2MeSATP reduced the V
max
of basal and SNP-stimulated
purified sGC activity by 65% and 77%, respectively (Fig. 5,
Table 2). 2MeSATP also increased the K
m
of purified basal
and SNP-stimulated sGC threefold and fourfold, respect-
ively [Table 2]. Employing Mn
2+
as the cation cofactor,
2MeSATP decreased the V
max
of purified sGC by 80% and
increased the K
m
1.5-fold (Fig. 6, Table 2). These char-
acteristics, including a decrease in V
max
and increase in K
m
,
suggest that 2-substituted adenine nucleotides inhibit puri-
fied sGC by a mixed noncompetitive mechanism, consistent
with allosteric regulation. These results are nearly identical
to those obtained examining the regulation of GC-C by
2-substituted nucleotides [19].
Regulation of GC-C by 2-substituted adenine nucleotides
is guanine-nucleotide dependent, and increasing concentra-
tions of GTP increase the potency of 2MeSATP and
2ClATP to inhibit GC-C [26]. Thus, the effect of guanine
nucleotides on the inhibition of purified sGC by 2-substi-
tuted nucleotides was examined. 2MeSATP inhibited GC-C
in T84 human colon carcinoma cells (Fig. 2A) and the
potency of that nucleotide to induce inhibition was
increased nearly eightfold by increasing concentrations of
guanine nucleotide from 10 to 100 l
M
, consistent with
previous observations (Table 3) [26]. At concentrations
>100 l
M
, GTP inhibited GC-C (data not shown) [26]. In
contrast, increasing concentrations of GTP from 10 to
Table 1. K
i
values for 2MeSATP and 2ClATP inhibition of crude and purified sGC. Guanylate cyclase was assayed in the presence of increasing
concentrations of the indicated nucleotide, 1 m
M
GTP, and 3 m
M
excess metal cation. Values ± SEM were determined from nonlinear regression
analysis of the sigmoidial plots from three separate experiments. ND, not determined.
sGC Nucleotide
K
i
± SEM (l
M
)
Mg
2+
Mg
2+
+50l
M
SNP Mn
2+
Crude 2MeSATP 570 ± 240 767 ± 158 ND
2ClATP 457 ± 57 282 ± 66 ND
Purified 2MeSATP 561 ± 166 460 ± 119 53 ± 24
2ClATP 370 ± 136 99 ± 31 42 ± 22
Fig. 4. Effect of adenine nucleotides on purified sGC activity using
Mn
2+
as the substrate cation cofactor. Guanylate cyclase activity was
measured in the presence of increasing concentrations of 2MeSATP
(h)or2ClATP(m), 1 m
M
MnGTP, and 3 m
M
Mn
2+
in excess of
nucleotides. Enzyme activities are expressed as fractional response as
described in Fig. 3. Basal activity of purified sGC using Mn
2+
as the
substrate cofactor was 369 ± 47 nmol cGMP min
)1
Æmg
)1
of protein.
Nonlinear regression analysis of the sigmoidial plots for each of the
nucleotides was used to estimate the K
i
values presented in Table 1.
Fig. 5. Effect of 2MeSATP on the relationship between activity and
substrate concentration of (A) basal and (B) SNP-stimulated purified
sGC using Mg
2+
as the substrate cation cofactor. Purified sGC activity
was quantified, using a range of substrate concentrations in the pres-
ence or absence of 2MeSATP with MgCl
2
as the substrate cation
cofactor, employing Michaelis (left) and Lineweaver–Burke (right)
plots analysis. Open circles, no addition; closed squares, 1 m
M
2MeSATP; open triangles, 50 l
M
SNP; closed diamonds, 50 l
M
SNP + 1 m
M
2MeSATP.
FEBS 2002 Adenine nucleotides directly inhibit sGC (Eur. J. Biochem. 269) 2189
100 l
M
did not increase the potency of 2MeSATP to induce
inhibition and concentrations of GTP > 100 l
M
,didnot
directly inhibit sGC (Fig. 6, Table 3).
DISCUSSION
Regulation of receptor–effector coupling and effector
response by purine nucleotides is a general mechanism
regulating transmembrane signaling by nucleotide cyclases.
Seven-transmembrane-domain receptors are coupled to
adenylate cyclase and cAMP production by heterotrimeric
guanine nucleotide-binding (G) proteins. In this system,
ligand–receptor interaction induces exchange of GDP for
GTP by G proteins which activate their coupling function,
permitting receptor-coupled regulation of adenylate cyclase
and accumulation of [cAMP]
i
. In addition, the catalytic
domains of adenylate cyclases are allosterically regulated by
adenine nucleotides. Thus, adenine nucleotides, including
2¢,5¢-dideoxy-3¢ATP and 2¢,5¢-dideoxy-3¢ADP, inhibit crude
and purified adenylate cyclases by a noncompetitive or
uncompetitive mechanism [27–30]. These nucleotides are
thought to bind directly to the C
1
–C
2
interface of the
catalytic domain of adenylate cyclase, the P site, which
mediates allosteric inhibition [28]. P site effectors inhibit
forskolin-, G
sa
-, or Mn
2+
-stimulated adenylate cyclase
[31–34]. Although 2¢,5¢-dideoxy-3¢ATP and 2¢,5¢-dideoxy-
3¢ADP are not natural products of cellular metabolism,
recent studies suggest that 2¢-deoxyadenosine 3¢-polyphos-
phates might be the natural allosteric effectors for P site
regulation of adenylate cyclases [27].
Regulation of guanylate cyclases by purine nucleotides
also is complex. Coupling between the ligand binding and
catalytic domains of pGCs is mediated by the KHD in the
cytoplasm, which serves as a constitutive repressor of the
catalytic domain. This domain contains the 11 subdomains
characteristic of protein kinases, but lacks the critical
aspartate residue in subdomain VI required for phospho-
transferase activity [35]. Ligand–receptor interaction induces
Fig. 6. Effect of 2MeSATP on the relationship between activity and
substrate concentration of purified sGC using Mn
2+
as the substrate
cation cofactor. (A) Michaelis plot of purified sGC in the presence of
2MeSATP. Guanylate cyclase activity was quantified using a range
of substrate concentrations in the presence or absence of 1 m
M
2MeSATP with Mn
2+
as the substrate cation cofactor. Open circles,
1m
M
MnGTP; closed squares, 1 m
M
MnGTP + 1 m
M
2MeSATP.
(B) Double-reciprocal plot of the data presented in panel (A). Open
circles, 1 m
M
MnGTP; closed squares, 1 m
M
MnGTP + 1 m
M
2MeSATP.
Table 3. Effect of GTP on the potency of 2-substituted adenine
nucleotides to inhibit purified sGC. ND, not determined.
GTP (l
M
)
K
i
± SEM
GC-C Purified sGC
10 88.2 ± 5.9 12.5 ± 3.6
20 ND 11.0 ± 4.0
50 ND 16.5 ± 2.5
100 12.1 ± 2.7
a
38.2 ± 9.6
a
< 0.05 vs. the K
i
value of 2MeSATP at 10 l
M
GTP for GC-C.
Table 2. Effect of 2-substituted adenine nucleotides on the kinetic parameters of purified sGC. Guanylate cyclase was assayed in the presence of
increasing concentrations of MgGTP (10 l
M
)10 m
M
)orMnGTP(3.9l
M
to 1 m
M
) in the presence or absence of 2MeSATP. The V
max
and K
m
were determined by nonlinear regression analysis of Michaelis plots. Values ± SEM are representative of three experiments. ND, not determined.
Agonist
MgGTP MnGTP
V
maxa
K
m
(l
M
)V
maxa
K
m
(l
M
)
Basal 181 ± 55 0.50 ± 0.18 311 ± 93 13 ± 5.5
2MeSATP 65 ± 24 1.36 ± 0.33 67 ± 0.06 17 ± 6.2
SNP 2711 ± 1079 0.07 ± 0.01 ND ND
SNP + 2MeSATP 630 ± 190 0.28 ± 0.11 ND ND
a
Nanomoles of cGMP produced per minÆmg
)1
of protein.
2190 I. Ruiz-Stewart et al. (Eur. J. Biochem. 269)FEBS 2002



















![Bộ Thí Nghiệm Vi Điều Khiển: Nghiên Cứu và Ứng Dụng [A-Z]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/10301767836127.jpg)
![Nghiên Cứu TikTok: Tác Động và Hành Vi Giới Trẻ [Mới Nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/24371767836128.jpg)





