GROUP VIIA
The Halogens
Halogens (Greek hals, “salt”; genes, “born”)
1.Tính chất lý học
2.Tính chất hóa học
3.Điều chế và ứng dụng
4.Hidro halogenua
5.Hợp chất chứa oxi của
halogen Department of Inorganic Chemistry - HUT
Department of Inorganic Chemistry - HUT
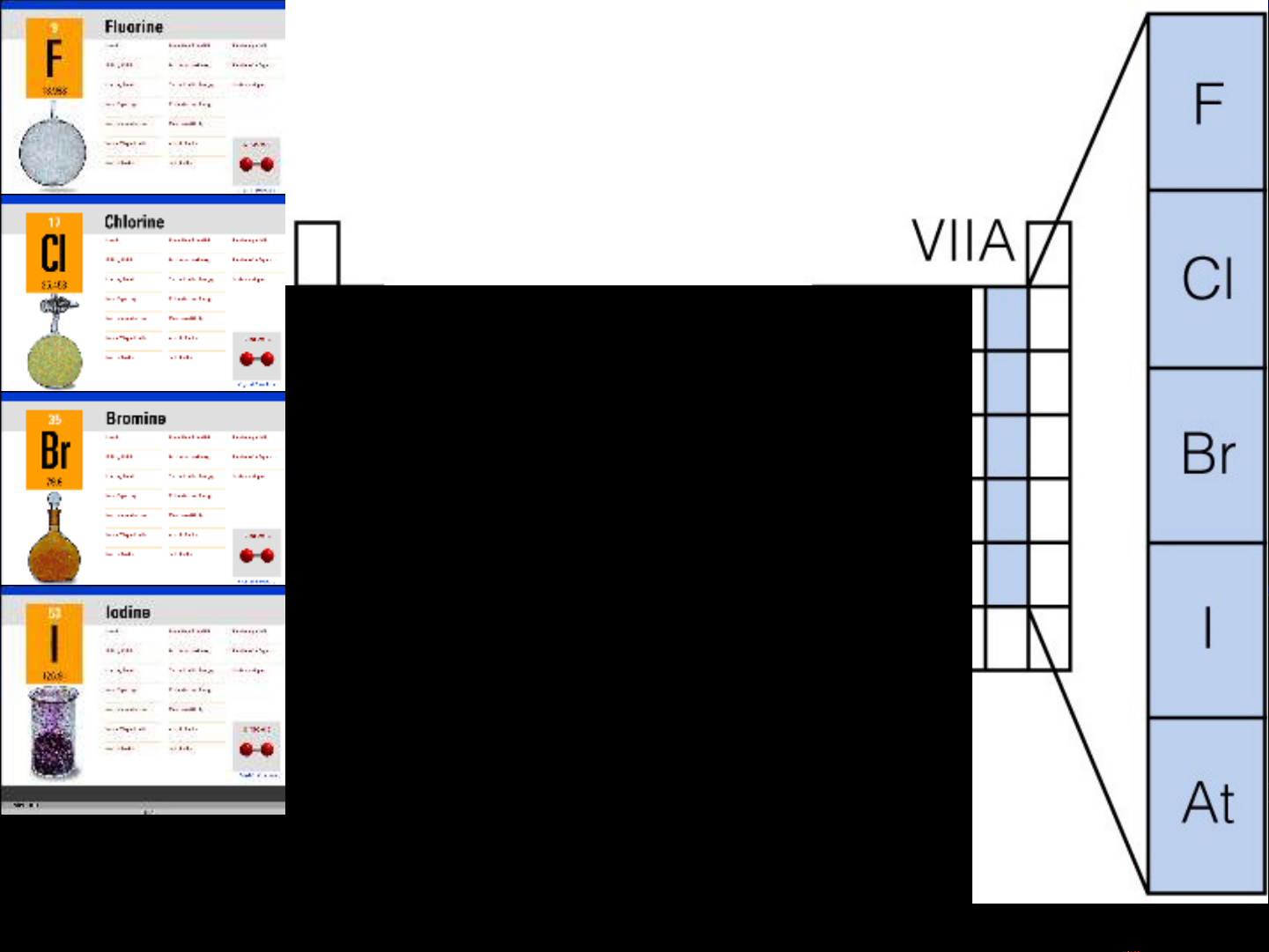
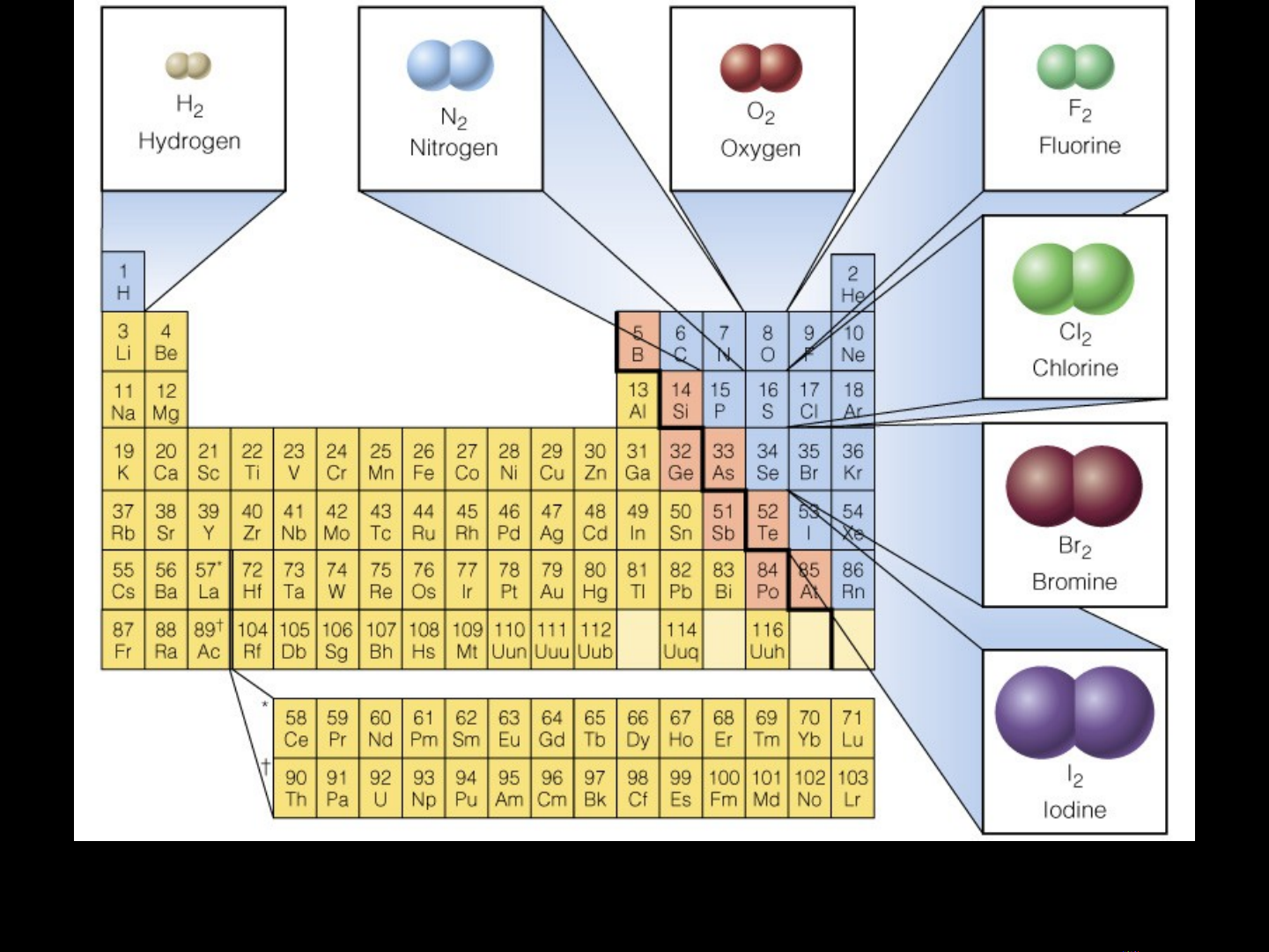
Group 17 Elements
Also known as Group VIIA
Halogens
Nonmetals:
Nonmetals:
Fluorine (F) and Chlorine (Cl) are gases
Bromine (Br) is a liquid
Iodine (I) is a solid
Metalloid:
Metalloid:
Astatine (At) is a solid

Department of Inorganic Chemistry - HUT
Department of Inorganic Chemistry - HUT
2 *2 2 2 2 *2 *2
s s z x y x y
σ σ σ π π π π
= =

Department of Inorganic Chemistry - HUT
The Lewis dot structure
Xns
ns2
2np
np4
4nd
nd1
1:
: ClF
ClF3
3
ns
ns2
2np
np5
5ns
ns2
2np
np3
3nd
nd2
2:
: BrF
BrF5
5
ns
ns2
2np
np2
2nd
nd3
3:
: IF
IF7
7













![Đề thi kết thúc học phần Nguyên lí Hóa học 2 [mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20251014/anhinhduyet000/135x160/69761760428591.jpg)












