
The Janus-faced atracotoxins are specific blockers
of invertebrate K
Ca
channels
Simon J. Gunning
1
, Francesco Maggio
2,
*, Monique J. Windley
1
, Stella M. Valenzuela
1
,
Glenn F. King
3
and Graham M. Nicholson
1
1 Neurotoxin Research Group, Department of Medical & Molecular Biosciences, University of Technology, Sydney, Australia
2 Department of Molecular, Microbial & Structural Biology, University of Connecticut School of Medicine, Farmington, CT, USA
3 Division of Chemical and Structural Biology, Institute for Molecular Bioscience, University of Queensland, Brisbane, Australia
The Janus-faced atracotoxins (J-ACTXs) are a novel
family of excitatory neurotoxins isolated from the
venom of the deadly Australian funnel-web spider [1].
In addition to their unusual pharmacology, these
peptide toxins are structurally unique: in addition to
having an inhibitory cystine knot motif that is common
to peptide toxins [2,3], they contain a rare and function-
ally critical vicinal disulfide bridge between adjacent
amino acids [1] (See Fig. 1).
The J-ACTXs are lethal to a wide range of inver-
tebrates, including flies, crickets, mealworms, and
budworms, but are inactive in mice, chickens, and
rats [1,4–6]; the molecular target of the J-ACTXs
has remained elusive ever since their discovery. The
insect specificity and excitatory phenotype of J-ACTX-
Hv1c are reminiscent of a subclass of scorpion b-toxins
that target insect voltage-activated Na
+
(Na
v
) channels
[7]. In addition, the 3D structure of J-ACTX-Hv1c
Keywords
alaine-scan mutants; bioinsecticide; BK
Ca
channel; cockroach neurons; kappa-
atracotoxin
Correspondence
G. M. Nicholson, Department of Medical
& Molecular Biosciences, University of
Technology, Sydney, PO Box 123,
Broadway NSW 2007, Australia
Fax: +61 2 9514 2228
Tel: +61 2 9514 2230
E-mail: Graham.Nicholson@uts.edu.au
*Present address
Bristol-Myers Squibb, Syracuse, NY, USA
(Received 6 May 2008, accepted 10 June
2008)
doi:10.1111/j.1742-4658.2008.06545.x
The Janus-faced atracotoxins are a unique family of excitatory peptide
toxins that contain a rare vicinal disulfide bridge. Although lethal to a wide
range of invertebrates, their molecular target has remained enigmatic for
almost a decade. We demonstrate here that these toxins are selective, high-
affinity blockers of invertebrate Ca
2+
-activated K
+
(K
Ca
) channels. Janus-
faced atracotoxin (J-ACTX)-Hv1c, the prototypic member of this toxin
family, selectively blocked K
Ca
channels in cockroach unpaired dorsal med-
ian neurons with an IC
50
of 2 nm, but it did not significantly affect a wide
range of other voltage-activated K
+
,Ca
2+
or Na
+
channel subtypes.
J-ACTX-Hv1c blocked heterologously expressed cockroach large-conduc-
tance Ca
2+
-activated K
+
(pSlo) channels without a significant shift in the
voltage dependence of activation. However, the block was voltage-depen-
dent, indicating that the toxin probably acts as a pore blocker rather than
a gating modifier. The molecular basis of the insect selectivity of J-ACTX-
Hv1c was established by its failure to significantly inhibit mouse mSlo
currents (IC
50
10 lm) and its lack of activity on rat dorsal root ganglion
neuron K
Ca
channel currents. This study establishes the Janus-faced atraco-
toxins as valuable tools for the study of invertebrate K
Ca
channels and
suggests that K
Ca
channels might be potential insecticide targets.
Abbreviations
4-AP, 4-aminopyridine; ACTX, atracotoxin; BK
Ca
channel, large-conductance Ca
2+
-activated K
+
channel; Ca
V
channel, voltage-activated Ca
2+
channel; ChTx, charybdotoxin; DRG, dorsal root ganglia; dSlo, Drosophila Slowpoke; DUM, dorsal unpaired median; hSlo, human slowpoke;
IbTx, iberiotoxin; IK
Ca
channel, intermediate-conductance K
Ca
channel; J-ACTX, Janus-faced atracotoxin; K
A
channel, transient ‘A-type’ K
+
channel; K
Ca
channel, Ca
2+
-activated K
+
channel; K
DR
channel, delayed-rectifier K
+
channel; K
V
channel, voltage-activated K
+
channel; mSlo,
mouse Slowpoke; Na
V
channel, voltage-activated Na
+
channel; NIS, normal insect saline; pSlo, Periplaneta Slowpoke; rSlo, rat Slowpoke;
SK
Ca
channel, small-conductance Ca
2+
-activated K
+
channel channel; TEA, tetraethylammonium; TTX, tetrodotoxin.
FEBS Journal 275 (2008) 4045–4059 ª2008 The Authors Journal compilation ª2008 FEBS 4045

resembles that of the excitatory Na
V
channel modu-
lator d-ACTX-Hv1a from the funnel-web spider
Hadronyche versuta [8]. However, Na
V
channels cannot
be the primary target of the J-ACTXs, as they are
active against the nematode Caenorhabditis elegans
(G. F. King, unpublished results), which does not
possess Na
V
channels [9].
In this study, we used patch clamp analysis of cock-
roach dorsal unpaired median (DUM) neurons to
determine the molecular target of the J-ACTXs. We
demonstrate that J-ACTX-Hv1c is a high-affinity
blocker of insect large-conductance Ca
2+
-activated
K
+
channel (BK
Ca
) currents, whereas it has minimal
effect on mouse or rat BK
Ca
channels. This work
establishes the J-ACTXs as valuable tools for the study
of invertebrate BK
Ca
channels, and it indicates that
insect BK
Ca
channels might be useful targets for the
development of novel insecticides.
Results
Specificity of J-ACTX-Hv1c action
Because of its structural homology to d-ACTX-Hv1a,
the lethal toxin from Australian funnel-web spiders that
delays inactivation of both vertebrate and invertebrate
voltage-activated Na
+
channels (Na
V
channels) [8,10],
we examined whether J-ACTX-Hv1c modulates Na
V
channel currents in cockroach DUM neurons. Test
pulses to )10 mV elicited a fast activating and inactivat-
ing inward Na
V
channel current (I
Na
) in DUM neurons
that could be abolished by addition of 150 nmtetrodo-
toxin (TTX). Subsequent exposure of isolated I
Na
to
1lmJ-ACTX-Hv1c failed to alter peak current ampli-
tude, inactivation kinetics (Fig. 2A), or the voltage
dependence of activation (data not shown, n= 5).
Subsequently, the actions of the toxin were assessed on
global inward voltage activated Ca
2+
(Ca
V
) channel
current (I
Ca
) in cockroach DUM neurons [11]. The elic-
ited current was abolished by addition of 1 mmCdCl
2
,
confirming that currents were carried via Ca
v
channels.
Application of J-ACTX-Hv1c (1 lm) failed to inhibit
I
Ca
elicited by a range of depolarizing test pulses from
)80 to +20 mV (Fig. 2B, n= 5), or alter the voltage
dependence of Ca
V
channel activation (data not shown,
n= 5). This indicates that J-ACTX-Hv1c does not
affect invertebrate Ca
V
channels.
Effects of J-ACTX-Hv1c on voltage-activated K
+
channel (K
V
channel) currents
Macroscopic K
v
channel currents (I
K
s) values in DUM
neurons were recorded in isolation from I
Na
and I
Ca
by using 200 nmTTX and 1 mmCd
2+
, respectively.
Macroscopic I
K
s were elicited by 100 ms depolarizing
pulses to +40 mV (Fig. 2F, inset) before, and 10 min
after, perfusion with toxin. In contrast to the lack of
overt modulation of Ca
V
and Na
V
channels, 1 lm
J-ACTX-Hv1c inhibited macroscopic outward I
K
by
56±7%(n= 5, Fig. 2C). This block was not accom-
panied by a shift in the voltage dependence of activa-
tion (data not shown). Block of macroscopic outward
I
K
indicates that J-ACTX-Hv1c targets at least one of
the four distinct K
+
channel subtypes identified in
DUM neuron somata [12]. These include delayed-recti-
fier K
+
channels (K
DR
channels), transient ‘A-type’
K
+
channels (K
A
channels), Na
+
-activated K
+
chan-
nels (K
Na
channels), and ‘late-sustained’ and ‘fast-tran-
sient’ Ca
2+
-activated K
+
channels (K
Ca
channels).
The fast-transient K
Ca
channel differs from the late-
sustained K
Ca
channel in that it inactivates rapidly
after activation and displays a voltage-dependent rest-
ing inactivation [13]. As a consequence of the inhibi-
tion of total I
K
, all subtypes except K
Na
channels were
investigated as potential targets of the J-ACTXs.
In order to isolate K
DR
channel currents [I
K(DR)
s] in
DUM neurons, K
A
channel curents [I
K(A)
s] were
blocked with 5 mm4-aminopyridine (4-AP) [13]. Addi-
tional experiments were required to determine the
concentration of charybdotoxin (ChTx) required to
block K
Ca
channel currents [I
K(Ca)
s] in DUM neurons.
Initial tests using 1 mmCdCl
2
produced only
35±7%(n= 7) inhibition of total outward I
K
in the
presence of 5 mm4-AP. Increasing concentrations of
ChTx in the presence of 1 mmCdCl
2
further inhibited
total outward I
K
in a concentration-dependent man-
ner. Addition of ChTx revealed a steep dose-response
relationship with inhibition of I
K
to 46 ± 5% at
30 nmand 46 ± 3% at 100 nm(n= 5), indicating
maximal inhibition of I
K(Ca)
at doses ‡30 nm
(Fig. 2D,E). This indicated that inhibition of Ca
2+
entry using CdCl
2
alone was insufficient to block total
I
K(Ca)
. Experiments requiring complete inhibition of
I
K(Ca)
, such as those involving I
K(DR)
and I
K(A)
, were
therefore performed with both 1 mmCdCl
2
and 30 nm
ChTx. Thus, outward I
K(DR)
could be recorded in
isolation from other I
K
channel subtypes by the addi-
tion of 1 mmCdCl
2
,5mm4-AP and 30 nmChTx.
J-ACTX-Hv1c (1 lm) did not inhibit I
K(DR)
(Fig. 2F,
n= 5) nor did it alter the voltage dependence of acti-
vation (n= 5, data not shown).
Neither I
K(A)
nor I
K(Ca)
can be recorded in isolation
from I
K(DR)
, as there are no selective blockers of insect
K
DR
channels [13]. Thus, I
K(A)
s were isolated using a
prepulse current-subtraction routine in the presence of
1mmCdCl
2
and 30 nmChTx to block I
K(Ca)
.I
K(DR)
s
Janus-faced atracotoxins block K
Ca
channels S. J. Gunning et al.
4046 FEBS Journal 275 (2008) 4045–4059 ª2008 The Authors Journal compilation ª2008 FEBS
were elicited in isolation from I
K(A)
by inactivating
I
K(A)
using a 1 s depolarizing prepulse to )40 mV fol-
lowed by a 100 ms test pulse to +40 mV (Fig. 2G,
inset). Currents recorded under these conditions were
digitally subtracted off-line from I
K(DR)
and I
K(A)
recorded with a prepulse potential to )120 mV. This
permitted isolation of I
K(DR)
from I
K(A)
. J-ACTX-
Hv1c (1 lm) produced a minor inhibition of I
K(A)
by
14 ± 4% (P< 0.05, n= 5) elicited by depolarizing
pulses to +40 mV (Fig. 2F). Again, J-ACTX-Hv1c
failed to alter the voltage dependence of activation
(data not shown, n= 5).
To record I
K(Ca)
in isolation from other K
V
channel
currents, a current-subtraction routine following perfu-
sion with the K
Ca
channel blockers CdCl
2
and ChTx
was utilized. Control macroscopic I
K(DR)
and I
K(Ca)
were elicited in the presence of 5 mm4-AP to block
I
K(A)
. J-ACTX-Hv1c was then perfused for a period of
10 min or until equilibrium was reached. CdCl
2
(1 mm) and ChTx (30 nm) were then added to block
K
Ca
channels. Residual K
DR
channel currents recorded
in the presence of the I
K(Ca)
blockers were then digi-
tally subtracted from both controls and currents
recorded in the presence of J-ACTX-Hv1c (Fig. 2G) to
A
B
C D E
F G
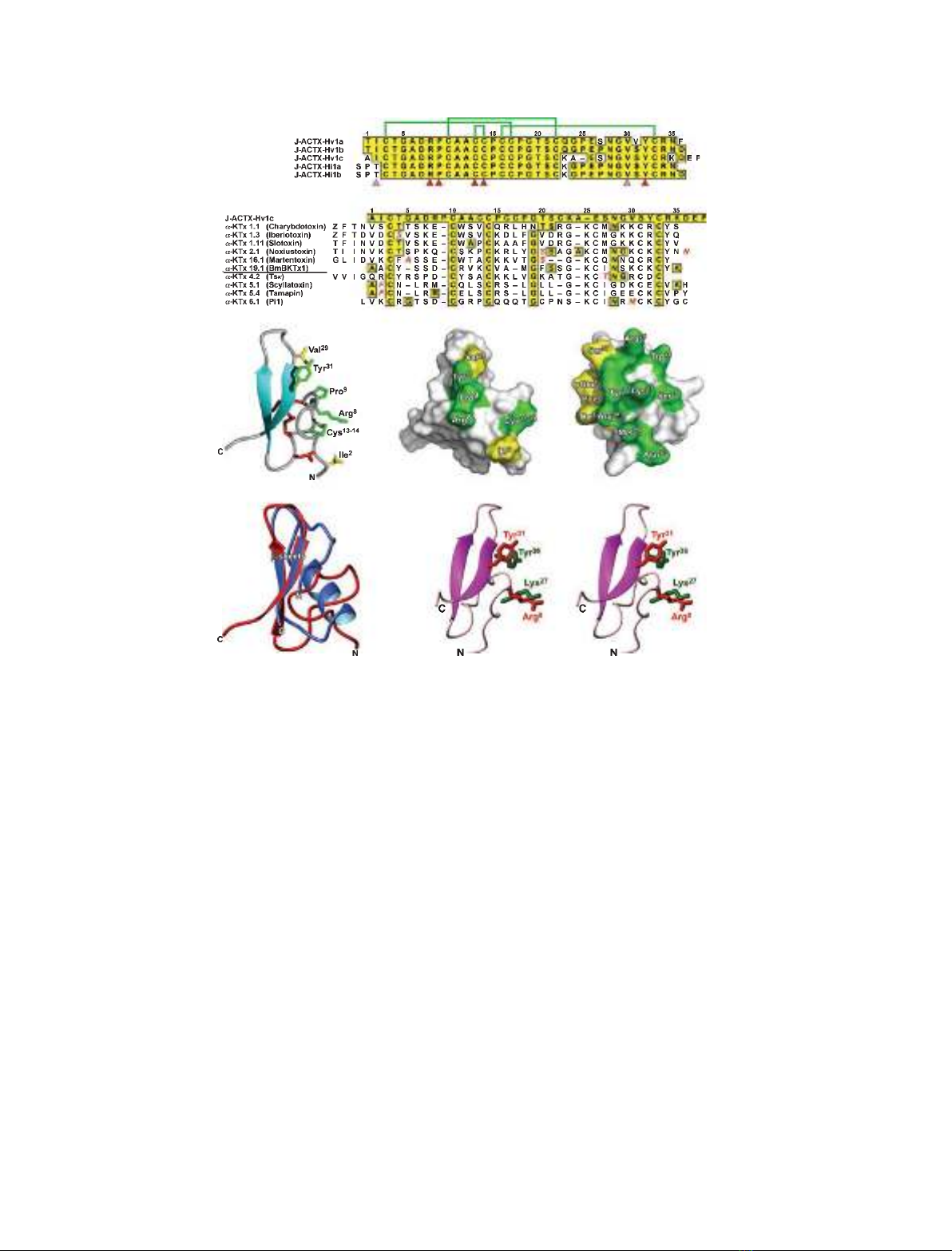
Fig. 1. Structure of J-ACTX-Hv1c and comparison with other BK
Ca
blockers. (A) Primary structure of J-ACTX-1 family members. Identities
are boxed in yellow. Green lines above the sequences represent the disulfide bonding pattern, and the arrowheads below highlight the phar-
macophore (red) and proposed water-excluding gasket (pink) residues of J-ACTX-Hv1c. (B) Comparison of the primary structure of J-ACTX-
Hv1c with known BK
Ca
(K
Ca
1.x) and SK
Ca
(K
Ca
2.x) channel blockers. Only toxins with nanomolar affinity for K
Ca
channels are included. Toxins
listed above the BmBKTx1 sequence are BK
Ca
channel blockers, and those below are SK
Ca
channel blockers. (C) Schematic of the structure
of J-ACTX-Hv1c (Protein Data Bank code 1DL0) highlighting the sidechains of the key pharmacophore residues (green) as well as those that
are proposed to serve as a water-excluding ‘gasket’ (see text for details). Disulfide bonds and b-strands are shown in red and cyan, respec-
tively. (D, E) Surface representation of J-ACTX-Hv1c (D) and ChTx (E), highlighting the primary pharmacophore residues. In the case of ChTx
(a-KTx 1.1), six of the eight residues crucial for activity on BK
Ca
channels are located on the b-strands. Pharmacophore and gasket residues
are shown in green and yellow, respectively. (F) Overlay of the structure of J-ACTX-Hv1c (red) and ChTx (Protein Data Bank code 2CRD,
blue). (G) Stereoview of an overlay of the functional dyad of ChTx (green side chains) with the ‘pseudo-dyad’ of J-ACTX-Hv1c (red side
chains). Only the backbone of J-ACTX-Hv1c is shown, for the sake of clarity.
S. J. Gunning et al. Janus-faced atracotoxins block K
Ca
channels
FEBS Journal 275 (2008) 4045–4059 ª2008 The Authors Journal compilation ª2008 FEBS 4047
isolate I
K(Ca)
. This subtraction routine is valid, given
the distinct lack of activity of J-ACTX-Hv1c on
I
K(DR)
. Isolated I
K(Ca)
exhibited fast activation, but
inactivated in two phases. Initial inactivation resulted
in a fast-transient component, with a subsequent late-
maintained phase that displayed much slower inactiva-
tion kinetics. The I
K(Ca)
also activated at membrane
potentials greater than )50 mV. These characteristics
are classical for BK
Ca
channel currents recorded in
DUM neurons [12,13].
In contrast to the lack of overt actions on K
DR
and K
A
channels, J-ACTX-Hv1c produced a potent
block of I
K(Ca)
that was only partially reversible
following prolonged washout in toxin-free solution
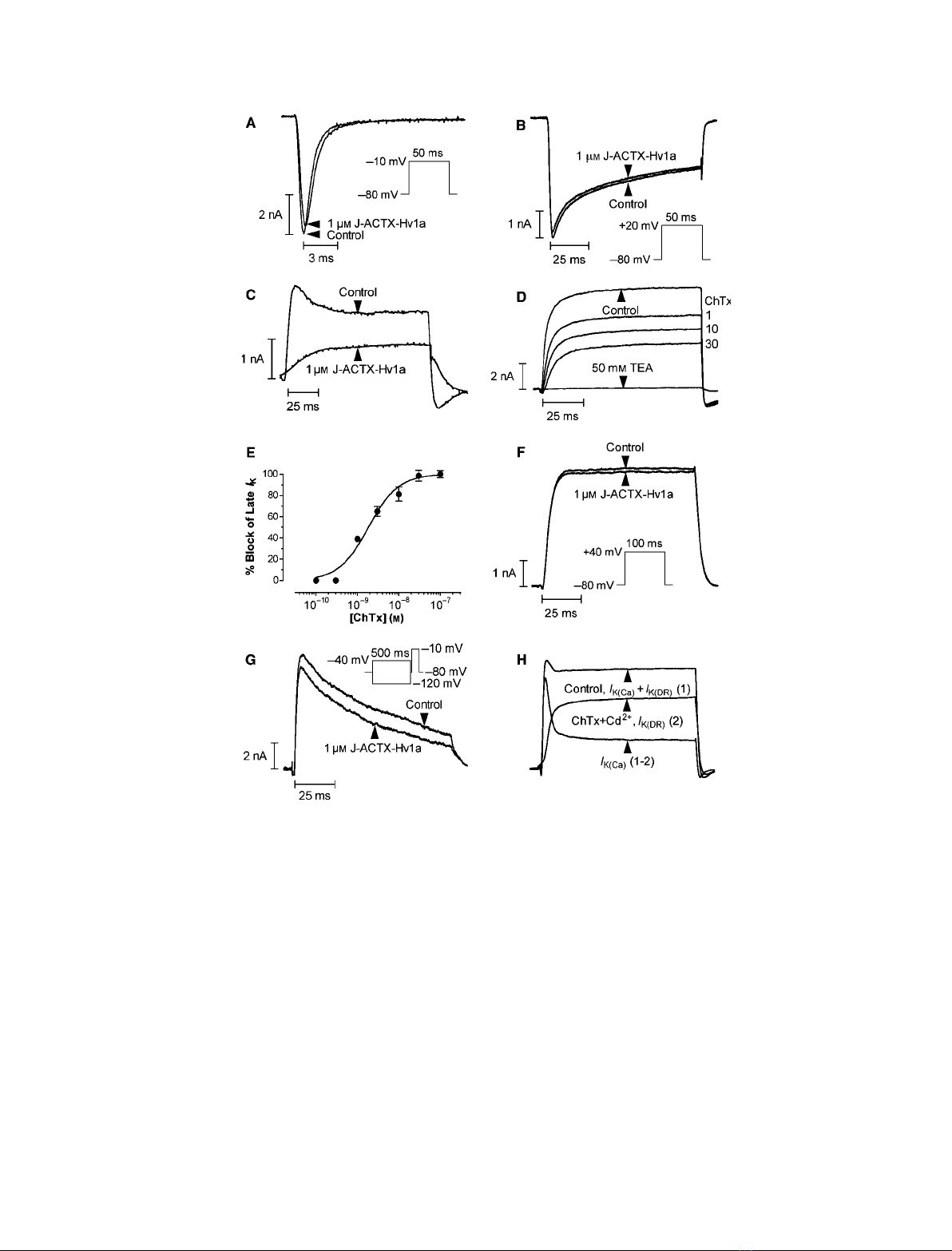
Fig. 2. Effect of J-ACTX-Hv1c on voltage-activated ion channels in cockroach neurons. (A, B) Superimposed current traces showing typical
lack of effect of 1 lMJ-ACTX-Hv1c on I
Ca
(A) and I
Na
(B). (C) Inhibition of macroscopic I
K
by 1 lMJ-ACTX-Hv1c. (D) Typical block of I
K(Ca)
by
increasing concentrations of ChTx (in nM). Subsequent addition of TEA in the presence of 30 nMChTx abolished the remaining current, thus
confirming that currents were carried by K
V
channels. Data were recorded from the same cell. (E) Dose–response curve for ChTx inhibition
of I
K(Ca)
recorded at the end of the pulse, in the presence of 1 mMCd
2+
(n= 5). (F, G) Typical effects of 1 lMJ-ACTX-Hv1c on I
K(DR)
(F) and
I
K(A)
(G). Superimposed I
K(A)
s were obtained by current-subtraction routines following prepulse potentials of )120 and )40 mV, shown in the
inset (see Experimental procedures). (H) Current-subtraction routine employed to isolate I
K(Ca)
(see Experimental procedures). The currents in
(C), (D), (F) and (H) were elicited by the test pulse protocol shown in the inset of (F).
Janus-faced atracotoxins block K
Ca
channels S. J. Gunning et al.
4048 FEBS Journal 275 (2008) 4045–4059 ª2008 The Authors Journal compilation ª2008 FEBS
(Fig. 3A). Inhibition of cockroach I
K(Ca)
was dose-
dependent, with IC
50
values of 2.3 nmand 2.9 nm,at
+40 mV, for the fast-transient and late-sustained
I
K(Ca)
, respectively (Fig. 3D). In order to further
examine the hypothesis that the target of J-ACTX-
Hv1c is an insect K
Ca
channel, we investigated
whether the toxin could produce an additional block
in the presence of maximal concentrations of ChTx.
Following inhibition of I
K
with 30 nmChTx, subse-
quent application of 1 lmJ-ACTX-Hv1c failed to
produce any additional block (Fig. 3E). In the com-
plementary experiment, 30 nmChTx failed to produce
any additional block of I
K
following inhibition of the
current with 1 lmJ-ACTX-Hv1c (Fig. 3F). These
findings provide further evidence that these peptides
act on the same molecular target in insect DUM
neurons, namely K
Ca
channels.
The effect of J-ACTX-Hv1c on I
K(Ca)
was inverte-
brate-selective, as the toxin failed to block either mac-
roscopic outward K
V
currents in rat dorsal root
ganglia (DRG) neurons (Fig. 3B, n=4) or I
K(Ca)
in
these neurons (Fig. 3C, n= 4) isolated using the same
current-subtraction routine as described earlier. Block
of I
K(Ca)
occurred without significant alteration of the
A D
BE
CF
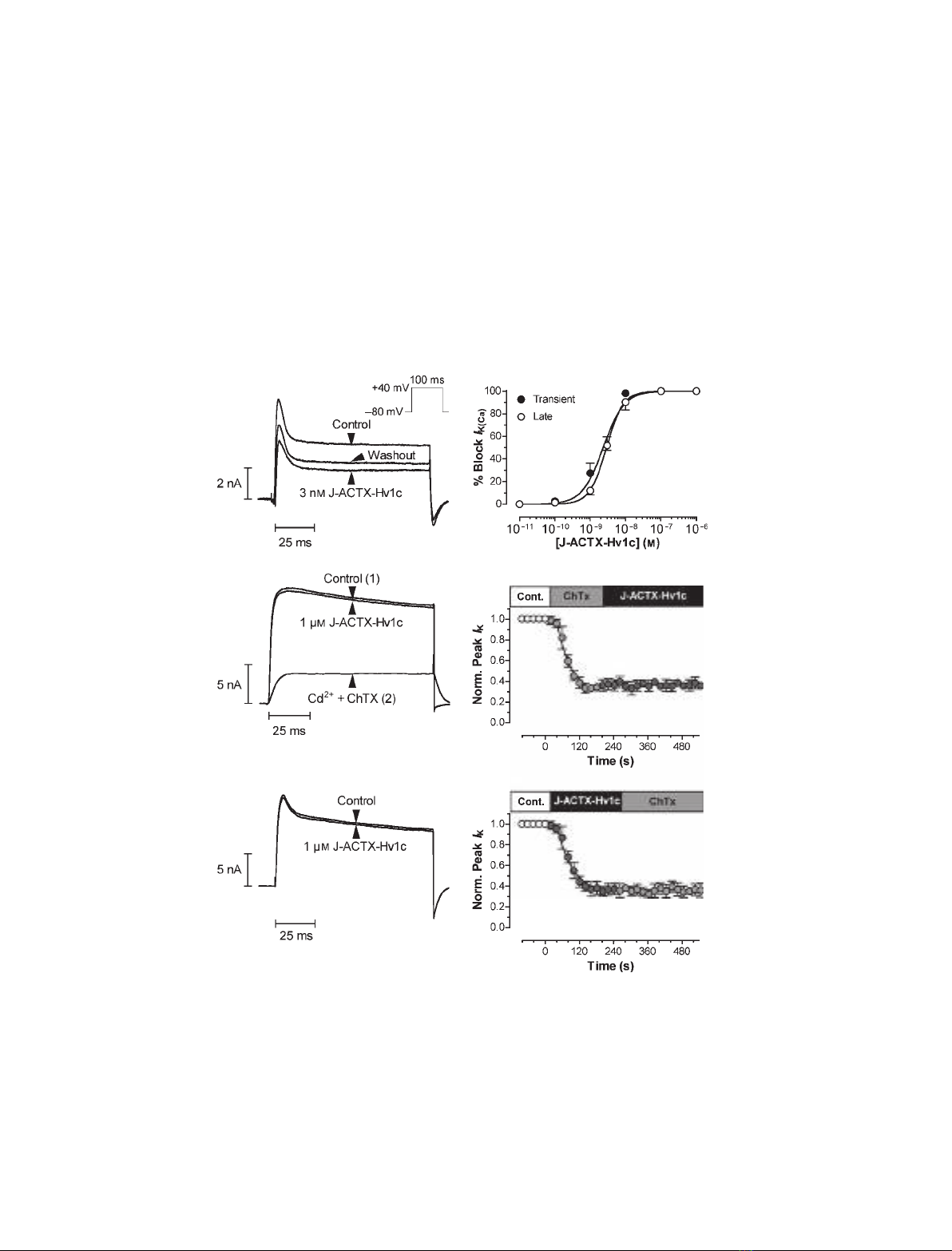
Fig. 3. J-ACTX-Hv1c blocks K
Ca
channels in cockroach DUM neurons. (A) Typical effects of 3 nMJ-ACTX-Hv1c on I
K(Ca)
, showing partial
reversibility. (B) Typical effect of 1 lMJ-ACTX-Hv1c on rat DRG neuron macroscopic I
K
. (C) J-ACTX-Hv1c (1 lM) failed to inhibit rat DRG
neuron I
K(Ca)
isolated by subtraction of the current remaining following addition of 100 nMChTx and 1 mMCd
2+
, shown in (B). (D) Dose–
response curve showing inhibition of I
K(Ca)
by J-ACTX-Hv1c in the presence of 1 mMCd
2+
(n= 3 at 1 lMand n= 5 at all other concentra-
tions). The currents in (A–D) were elicited by the test pulse protocol shown in the inset of (A). (E, F) J-ACTX-Hv1c and ChTx share the same
target in cockroach DUM neurons. (E) Addition of 1 lMJ-ACTX-Hv1c failed to further inhibit I
K
currents blocked by perfusion with 30 nM
ChTx and 1 mMCd
2+
(n= 5). (F) In the complementary experiment, addition of 30 nMChTx and 1 mMCa
2+
faile to further inhibit I
K
currents
blocked by perfusion with 1 lMJ-ACTX-Hv1c (n= 5). In both (E) and (F), currents were recorded in the presence of 4-AP to block I
K(A)
.
S. J. Gunning et al. Janus-faced atracotoxins block K
Ca
channels
FEBS Journal 275 (2008) 4045–4059 ª2008 The Authors Journal compilation ª2008 FEBS 4049


























