
Extrinsic proteins of photosystem II
An intermediate member of the PsbQ protein family in red algal PS II
Hisataka Ohta
1,2
, Takehiro Suzuki
1
, Masaji Ueno
1
, Akinori Okumura
1
, Shizue Yoshihara
1
, Jian-Ren Shen
3
and Isao Enami
1
1
Department of Biology, Faculty of Science and
2
Tissue Engineering Research Center, Tokyo University of Science, Japan;
3
Department of Biology, Faculty of Science, Okayama University and PRESTO, JST, Japan
The oxygen-evolving photosystem II (PS II) complex of red
algae contains four extrinsic proteins of 12 kDa, 20 kDa,
33 kDa and cyt c-550, among which the 20 kDa protein is
unique in that it is not found in other organisms. We cloned
the gene for the 20-kDa protein from a red alga Cyanidium
caldarium. The gene consists of a leader sequence which can
be divided into two parts: one for transfer across the plastid
envelope and the other for transfer into thylakoid lumen,
indicating that the gene is encoded by the nuclear genome.
The sequence of the mature 20-kDa protein has low but
significant homology with the extrinsic 17-kDa (PsbQ)
protein of PS II from green algae Volvox Carteri and
Chlamydomonas reinhardtii, as well as the PsbQ protein of
higher plants and PsbQ-like protein from cyanobacteria.
Cross-reconstitution experiments with combinations of the
extrinsic proteins and PS IIs from the red alga Cy. calda-
rium and green alga Ch. reinhardtii showed that the
extrinsic 20-kDa protein was functional in place of the
green algal 17-kDa protein on binding to the green algal
PS II and restoration of oxygen evolution. From these
results, we conclude that the 20-kDa protein is the ancestral
form of the extrinsic 17-kDa protein in green algal and
higher plant PS IIs. This provides an important clue to
the evolution of the oxygen-evolving complex from pro-
karyotic cyanobacteria to eukaryotic higher plants. The
gene coding for the extrinsic 20-kDa protein was named
psbQ¢(prime).
Keywords: photosystem II; oxygen evolution; extrinsic
protein; psbQ; red alga.
1
Oxidation of water by photosystem II (PS II) is the source
of molecular O
2
, electrons, and protons in higher plants,
algae, and cyanobacteria. PS II is a multisubunit pigment–
protein complex containing intrinsic and extrinsic compo-
nents located in thylakoid membranes. More than 10
intrinsic, membrane-spanning proteins including CP47,
CP43, D1, D2, aand bsubunits of cytochrome b-559,
and the psbI gene product form the transmembrane core of
PS II. The extrinsic components are known to maintain and
optimize the stability and activity of the water oxidation site,
which is composed of a cluster of four manganese atoms
located close to the luminal surface of the transmembrane
domain and coordinated mainly by amino acids of the D1
protein [1–3]. The extrinsic domain of the oxygen-evolving
complex is composed of three proteins of 33 kDa, 23 kDa
and 17 kDa encoded by psbO, psbP, psbQ genes, respect-
ively, in PS II of green algae and higher plants (reviewed in
[4]). Among these three extrinsic components, the 33-kDa
manganese stabilizing protein (PsbO) is highly conserved
from prokaryotic cyanobacteria to eukaryotic higher plants,
while the 23-kDa and 17-kDa proteins are absent in PS II
from cyanobacteria and red algae, although a PsbQ-like
protein was recently reported to be associated with PS II
from Synechocystis sp. PCC 6803 [5]. Instead, cyanobacte-
rial PS II contains two other extrinsic proteins, PsbU
(12 kDa) and PsbV (cyt c-550), which functions to replace
to some extent the role of PsbP and PsbQ found in green
algae and higher plants [6].
Among photosynthetic organisms, red algae are one of
the most primitive eukaryotic algae phylogenetically closely
related to the prokaryotic oxygenic cyanobacteria. We have
found that the oxygen-evolving PS II complex purified from
aredalga,Cyanidium caldarium contained three extrin-
sic proteins of cyanobacteria-type, i.e. the 33-kDa, 12-kDa
proteins and cyt c-550 [7]. In addition to these three
proteins, the red algal PS II contained a fourth extrin-
sic protein of 20 kDa [7,8]. N-terminal amino acid sequence
of more than 30 residues of the protein revealed that it has
no significant homology with any known PS II polypeptides
[7], suggesting that it is a new extrinsic component of PS II.
Release-reconstitution experiments in red algal PS II
showedthatthe20-kDaproteincanbindtoPSIItoa
significant extent by itself, whereas the effective binding of
cyt c-550 and the 12-kDa protein requires the presence of
both the 33-kDa and 20-kDa proteins [8]. This is in contrast
to the situation found in cyanobacterial PS II where cyt c-
550 could bind to PS II essentially independently of the
binding of the 33-kDa protein, and where the homologous
Correspondence to I. Enami, Department of Biology,
Faculty of Science, Tokyo University of Science,
1-3 Kagurazaka, Shinjuku, Tokyo 162-8601, Japan.
Fax: +81 471 24 2150, Tel.: +81 471 24 1501 ex5022,
E-mail: enami@rs.noda.tus.ac.jp
Abbreviation: PS II, photosystem II.
Note: The sequence reported in this paper has been deposited in the
DDBJ database (accession No. AB111526)
(Received 8 July 2003, revised 21 August 2003,
accepted 29 August 2003)
Eur. J. Biochem. 270, 4156–4163 (2003) FEBS 2003 doi:10.1046/j.1432-1033.2003.03810.x

20-kDa protein was not found. These results suggest a
gradual change of the oxygen-evolving complex from
prokaryotic cyanobacteria to eukaryotic red algae and
higher plants. The unique 20-kDa protein found in red algal
PS II may provide insights into such changes.
In this work, we cloned the gene for the 20-kDa protein
from the red alga, Cy. caldarium,andcomparedits
sequence with those of other PS II extrinsic proteins. It
was shown that the 20-kDa protein is homologous to the
PsbQ protein found in green algal and higher plant PS IIs.
The 20-kDa gene was successfully expressed in Escherichia
coli, and cross-reconstitution with the recombinant 20-kDa
protein showed that this protein is functional in place of the
PsbQ protein in green algal PS II. These results provided
important clues to the evolution of oxygen-evolving com-
plex from cyanobacteria to higher plants.
Materials and methods
Preparations
PS II membranes of spinach were prepared according to
Berthold et al. [9]. The extrinsic proteins of PS II were
extractedwith1
M
CaCl
2
as described by Enami et al. [10].
Oxygen-evolving PS II from the red alga Cy. caldarium was
prepared according to Enami et al. [7], and suspended in
40 m
M
Mes pH 6.5, 10 m
M
CaCl
2
, 25% glycerol. The four
extrinsic proteins were released with 1
M
CaCl
2
-wash and
purified as described by Enami et al. [8], and finally dialysed
extensively against 40 m
M
Mes pH 6.5 and concentrated.
Green algal oxygen-evolving PS II and its extrinsic proteins
were prepared from Chlamydomonas reinhardtii as described
by Suzuki et al. in [11].
Cloning and sequence analysis of the extrinsic
20-kDa protein
The N-terminal sequence of the 20-kDa protein was
determined as described by Enami et al.[12],andthe
sequence obtained was as follows: AGEPKMSFFGA
DAPSSPFTYNEREGEPVYK. Based on this sequence,
the gene coding for the 20-kDa protein from Cy. calda-
rium was cloned by a two-step PCR method. First, two
sets of degenerate oligonucleotide primers corresponding
to N-terminal sequence of AGEPKM and GEPVYK were
synthesized and used to amplify a 90-bp cDNA fragment
by RT/PCR from a Cy. caldarium cDNA library. The
cDNA fragment was sequenced to confirm that it indeed
corresponded to the N-terminal sequence of the 20-kDa
protein. Based on this information, the second PCR step
was performed with the RACE procedure [13] using the
Marathon cDNA Amplification Kit (Clontech) by which
DNA fragments including the 5¢-and3¢-flanking regions
of the 20-kDa protein were amplified using primers newly
synthesized based on the N-terminal 90-bp cDNA frag-
ment. This second-step PCR resulted in 450-bp and
600-bp cDNA fragments from the 5¢-and3¢-RACE,
respectively. Sequencing of these cDNA fragments con-
firmed that they contained the cDNA for the 20-kDa
protein. These sequences were combined with the partial
sequence of the N-terminal part to yield the whole
sequence of the gene.
The PCR fragments obtained were inserted into the
plasmid pCRII (TA Cloning Kit, Invitrogen), and the DNA
sequences were determined by the method of Dye Deoxy
Terminator Cycle Sequencing with a DNA Sequencer
(Applied Biosystems, model 310).
Expression and purification of the recombinant
20-kDa protein
The whole gene encoding the mature 20-kDa protein was
cloned into the LIC site of plasmid pET-32Xa/LIC,
resulting in a fusion protein with thioredoxin and (His)
6
-
tag attached at its N-terminus [14,15]. The recombinant
protein was expressed with the host cell BL21 (Novagen)
and purified by His-bind affinity chromatography according
to the manufacturer’s instructions. The fusion protein was
treated with Factor Xa to cleave off the thioredoxin and
His-tag and then purified again by affinity column.
Reconstitution
Reconstitution experiments of CaCl
2
-washed PS II from
red and green algae with various combinations of extrinsic
proteins from different sources were performed according to
Enami et al. [8,10] and Suzuki et al. [11]. SDS/PAGE was
performed according to Ikeuchi and Inoue [16]. Oxygen
evolution was measured with a Clark-type oxygen electrode
at 25 Cwith0.4m
M
phenyl-p-benzoquinone (red alga)
or 2,6-dichloro-p-benzoquinone (green alga) as electron
acceptor.
Results
Cloning and sequence analysis of the 20-kDa protein
The DNA sequences obtained in the present study are
shown in Fig. 1. Two in-frame ATG codons were found
upstream of the N-terminal alanine residue of the mature
polypeptide, one at position 40 and the other at position 91.
The start codon for the 20-kDa protein gene was assigned at
the first ATG codon of nucleotide number 40, because this
site (AAAAATGTT) has a better match with the consensus
sequence for plant translation initiation than the second site
(CTTGATGAT) [17]. According to this assignment, the
resulting gene encodes a polypeptide of 218 amino acid
residueswithatotalmolecularmassof24028Da.Asthe
N-terminal part of the mature 20-kDa protein corresponds
to sequence starting at residue number 73, residues 1–72
serve as leader sequences. Hydropathy analysis (data not
shown) revealed that there are two characteristic domains in
this leader sequence. The first consists of residues 1–47 and
is enriched in basic, hydrophilic, as well as hydroxylated
residues; this is consistent with the characteristic features of
transit peptides for transport across the chloroplast envel-
ope [4] and suggests that this domain functions to direct the
transfer of the 20-kDa protein across the chloroplast
envelope. The second domain consists of residues 48–72
and has features characteristic of transit peptides for
transfer of proteins through the bacterial periplasmic
membranes and thylakoid membranes [4], because its
central part is enriched in hydrophobic residues and its C
terminus contains an alanine residue at position )1(thisis
FEBS 2003 Characterization of the fourth extrinsic protein in PS II (Eur. J. Biochem. 270) 4157

typically found in proteins transported across the periplas-
mic and thylakoid membranes). Thus, we conclude that the
20-kDa protein is encoded by the nuclear DNA in the red
alga. This is consistent with results of whole chloroplast
genome sequencing of the red algae Porphyra purpurea [18]
and Cy. caldarium RK1 [19], in which the gene coding for
the 20-kDa protein was not found in the plastid genome.
Cleavage of the transit peptides resulted in a mature
polypeptide of 146 amino acid residues with a calculated
molecular mass of 16 386 Da.
Blast analysis with the GenBank database showed a
significant homology of the 20-kDa protein gene with a
cDNA clone, AV34507 from a marine red alga Porphyra
yezoensis [20]. Unexpectedly, this analysis also gave low but
significant scores (53–64) with oxygen-evolving enhancer
(OEE) protein 3 (PsbQ) from green algae Volvox carteri [21]
and Ch. reinhardtii [22]. These results suggested that the
extrinsic 20-kDa protein in PS II from the red alga
Cy. caldarium is a homologue of one of the PS II extrinsic
proteins, PsbQ protein, in green algae. Recently, Kashino
et al. reported that the sll1638 gene product of cyanobac-
terium Synechocystis sp. PCC 6803 has a similarity to the
PsbQ protein and is associated with the cyanobacterial
PS II complex [5]. We thus aligned the red algal 20-kDa
protein sequence with the PsbQ-like protein from two
cyanobacteria, Synechocystis sp. PCC 6803 and Anabaena
sp. PCC7120, the PsbQ protein from green algae and higher
plants whose sequences are currently available, together
with the homologous 20-kDa protein from the red alga
P. yezoensis, using the global alignment algorithm
CLU-
STALW
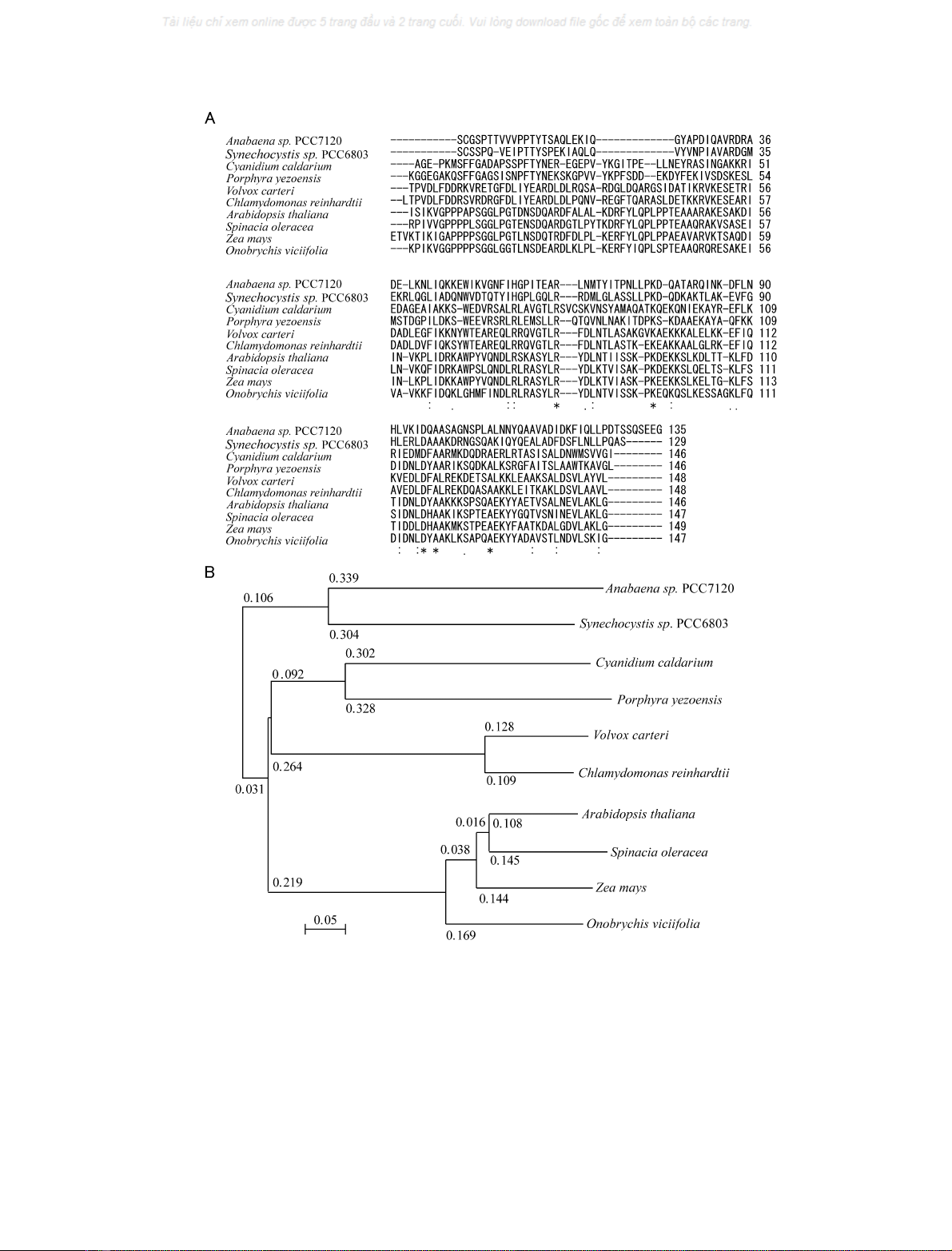
[23] (Fig. 2A). Based on these sequences, a phylo-
genetic tree was constructed by the neighbour-joining
algorithm as shown in Fig. 2B [24]. Generally, in contrast
with the other PS II extrinsic proteins such as the PsbO
protein which has a relatively high homology from cyano-
bacteria to higher plants, the PsbQ protein has a low
homology even between green algae and higher plants. For
example, the similarities (number of identical residues out of
the total residues) of the PsbO protein between cyanobac-
teria and higher plants range from 42% to 53% (Blast
similarity score, > 200), whereas those of the PsbQ protein
between the green algae Ch. reinhardtii or V. carteri and
spinach are 23% and 25% (Blast similarity score, 51–55),
respectively. In particular, the homology between the red
algal 20-kDa protein and the cyanobacterial PsbQ-like
protein is not high; blast analysis gave rise to a similarity
score less than 28 (20% identity). This is reminiscent of the
similarity between the cyanobacterial PsbQ-like protein and
higher plant PsbQ protein (Blast similarity score, < 39).
Consequently, the
CLUSTALW
multiple sequence alignment
shows that only five residues are completely conserved in the
C-terminal half of all sequences (Fig. 2A). Examination of
individual sequences showed that the 20-kDa protein
among red algae, and the PsbQ protein within the same
category of organisms are rather conserved. The resulting
phylogenic tree indicated that the PsbQ protein family could
be classified into four groups: (a) cyanobacteria; (b) red
algae; (c) green algae; and (d) higher plants. If we assume
that all these proteins were arisen from a common ancestral
protein, the PsbQ proteins of higher plants and green algae
were diverged at a very early stage from those of prokaryotic
cyanobacteria, whereas the red algal 20-kDa protein
remains rather unchanged. As a result, the red algal
20-kDa protein has a relatively low similarity with PsbQ
proteins from green algae and higher plants.
Reconstitution using the recombinant 20-kDa protein
For reconstitution experiments, the 20-kDa protein of
Cy. caldarium was successfully expressed as a fusion protein
with a His-tag using the pET expression system. The
expressed protein was purified by His-bind affinity chro-
matography, and the His-tag was proteolytically removed
by Factor Xa. This recombinant 20-kDa protein was used
for reconstitution experiments with the red algal PS II.
To compare the binding and functional properties of the
recombinant 20-kDa protein with those of the native
20-kDa protein, reconstitution experiments were first car-
ried out with the native 20-kDa protein purified from the
red algal PS II. As described previously [8,10], four extrinsic
Fig. 1. Nucleotide sequence of the 20-kDa extrinsic protein of PS II
from the red alga, Cyanidium caldarium.The deduced amino acid
sequence is shown below the nucleotide sequence in the single-letter
code. The putative chloroplast envelope transit domain (solid line) and
thylakoid transfer domain (dashed line) are underlined. Arrowhead
indicates the cleavage site generating the mature 20-kDa protein, and
the asterisk indicates the stop codon.
4158 H. Ohta et al. (Eur. J. Biochem. 270)FEBS 2003
proteins were completely released by treatment with 1
M
CaCl
2
of the purified PS II particles from Cy. caldarium
(Fig. 3, lane 2). The 12-kDa protein and cyt c-550 rebound
to the CaCl
2
-washed PS II efficiently when they were recon-
stituted together with the 33-kDa protein (Fig. 3, lane 3),
but their rebinding was not complete. Reconstitution
Fig. 2. Phylogenetic analysis of the 20-kDa protein sequence. (A) Alignment of the mature part of the 20-kDa protein sequence of Cyanidium
caldarium with a homologous protein from a marine red alga Porphyra yezoensis [20], and those of the PsbQ related proteins from two cyano-
bacteria Anabaena sp. PCC7120 [27] and Synechocystis sp. PCC6803 [28], two green algae Volvox Carteri (U22330) [21] and Chlamydomonas
reinhardtii [22], and four species of higher plants Spinacia oleracea [29], Arabidopsis thaliana [30], Zea mays [31], Onobrychis viciifolia (GenBank
Accession: AAB81994). The alignment was made with the global alignment algorithm
CLUSTAL X
[23]. Asterisks indicate identical residues among
all the sequences compared; double dots indicate conserved replacement of the residue in some of the species, and single dots indicate a slightly less
conserved replacement of the residue in some of the species. (B) Phylogenetic tree of the PsbQ protein family constructed based on the alignment
shown above. See text for further discussions.
FEBS 2003 Characterization of the fourth extrinsic protein in PS II (Eur. J. Biochem. 270) 4159
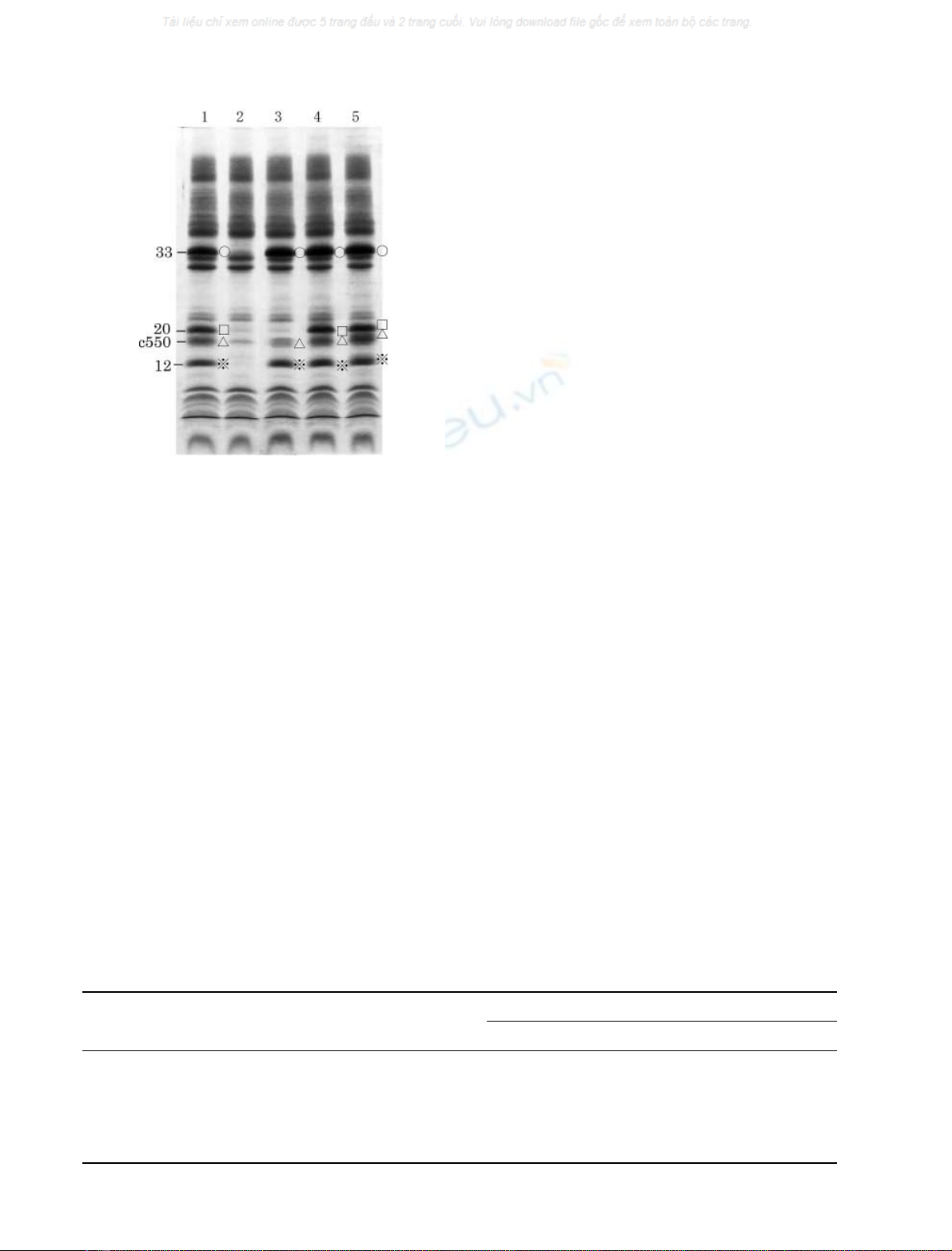
of these three extrinsic proteins together with the native
20-kDa protein resulted in a complete rebinding of all of the
four extrinsic proteins (Fig. 3, lane 4). Similarly, reconsti-
tution of the recombinant 20-kDa protein together with the
other three proteins also resulted in the complete rebinding
of the four extrinsic proteins (Fig. 3, lane 5). This indicates
that the recombinant 20-kDa protein retained the same
binding ability as that of the native 20-kDa protein.
Table 1 shows the restoration of oxygen evolution of the
CaCl
2
-washed PS II upon reconstitution with the extrinsic
proteins. The native PS II of Cy. caldarium showed a high
activity of 2754 lmol O
2
Æmg chl
)1
Æh
)1
2in the absence of
NaCl in the assay medium; this activity did not increase
much upon supplemention by NaCl. Upon CaCl
2
-wash, no
activity was observed in the absence or presence of NaCl.
Reconstitution with all the four native proteins increased
the activity to 50% and 51% of that in the native PS II,
respectively, in the absence and presence of NaCl. Recon-
stitution with the recombinant 20-kDa protein together with
the other three native proteins restored the oxygen-evolving
activity to a similar level as that with the native 20-kDa
protein, indicating that the recombinant protein was
functional in the red algal PS II and was as effective as
the native protein.
Cross-reconstitution of the 20-kDa protein and green
algal extrinsic 17-kDa protein
As the 20-kDa protein from Cy. caldarium has a sequence
homology with higher plant PsbQ protein, we tried to cross-
reconstitute the 20-kDa protein to well characterized
spinach PS II in place of the 17-kDa protein. However,
the 20-kDa protein was neither able to bind to CaCl
2
-
washed spinach PS II in the presence of the spinach extrinsic
33-kDa and 23-kDa proteins nor contributed to increase of
the Cl
–
binding affinity for oxygen evolution (data not
shown). Recently, we have purified oxygen-evolving PS II
complexes from a green alga, Ch. reinhardtii having His-
tagged CP47, and reported that the extrinsic 17-kDa protein
of Ch. reinhardtii directly bound to PS II independently of
the other extrinsic proteins [11], which is apparently in
contrast with the spinach 17-kDa protein which functionally
associates with PS II only through its interaction with both
the 33-kDa and 23-kDa proteins [25]. This binding property
of the 17-kDa protein in green algal PS II is similar to that
of the 20-kDa protein in the red algal PS II in that the latter
also binds to PS II by itself and promotes the complete
binding of the 12-kDa protein and cyt c-550totheredalgal
PS II [8]. Thus, we performed cross-reconstitution experi-
ments between the 20-kDa protein from the red alga and the
17-kDa protein from the green alga, with PS IIs from both
redandgreenalgae.
First, we examined whether the green algal 17-kDa
protein is exchangeable for the 20-kDa protein in binding
to the red algal PS II. The resulting PS II was analysed
by SDS/PAGE (Fig. 4A). In agreement with the results
obtained in Fig. 3, significant amounts of the 12-kDa
protein and cyt c-550 bound to CaCl
2
-washed PS II from
the red alga in the presence of the 33-kDa protein, but the
20-kDa protein was essential for complete binding of the
12-kDa protein and cyt c-550 (Fig. 4A, lanes 1 and 2).
When the 20-kDa protein was replaced by the green algal
extrinsic 17-kDa protein, the 17-kDa protein was able to
bind to the red algal PS II to a moderate level, but this
binding scarcely enhanced the binding of 12-kDa protein
and cyt c-550 (Fig. 4A, lane 3). These results agree with the
restoration of oxygen evolution which showed a decreased
Cl
–
requirement upon reconstitution with the 20-kDa
Fig. 3. Reconstitution of CaCl
2
-treatedPSIIoftheredalgawitheither
the native 20-kDa protein or the recombinant 20-kDa protein, in com-
binations with other three native extrinsic proteins of 33 kDa, 12 kDa
and cyt c-550. Lane 1, control PS II; lane 2, CaCl
2
-treated PS II; lanes
3–5, CaCl
2
-treated PS II reconstituted with the three extrinsic proteins
of 33 kDa, 12 kDa and cyt c-550 (lane 3), with the three extrinsic
proteins plus the native 20 kDa protein (lane 4), and with the three
extrinsic proteins plus the recombinant 20 kDa protein (lane 5).
Table 1. Restoration of oxygen evolution of CaCl
2
-treated red algal PS II by reconstitution with native or recombinant extrinsic 20-kDa protein.
Oxygen evolving activity (lmol O
2
Æmg chl
)1
Æh
)1
)
–
ion (%) +10 m
M
NaCl (%)
Cyanidium PS II 2754 ± 21 (100) 2756 ± 31 (100)
CaCl
2
-treated PS II 0 (0) 0 (0)
+ 33 0 (0) 496 ± 22 (18)
+ 33 + cyt c-550 + 12 1157 ± 18 (42) 1350 ± 40 (49)
+ 33 + cyt c-550 + 12 + native 20 1378 ± 30 (50) 1402 ± 27 (51)
+ 33 + cyt c-550 + 12 + recombinant 20 1406 ± 16 (51) 1433 ± 38 (52)
4160 H. Ohta et al. (Eur. J. Biochem. 270)FEBS 2003


























