
Emergence of a subfamily of xylanase inhibitors within
glycoside hydrolase family 18
Anne Durand
1,
*, Richard Hughes
1,
*, Alain Roussel
2
, Ruth Flatman
1,
*, Bernard Henrissat
2
and Nathalie Juge
1,3
1 Institute of Food Research (IFR), Norwich, UK
2 Architecture et Fonction des Macromole
´cules Biologiques, UMR6098, CNRS et Universite
´s d’Aix-Marseille I et II, Marseille, France
3 Institut Me
´diterrane
´en de Recherche en Nutrition, UMR INRA 1111, Faculte
´des Sciences et Techniques de St Je
´ro
ˆme, Marseille, France
Recently two classes of plant proteins, designated as
XIP (xylanase inhibitor protein) [1] and TAXI (Triti-
cum aestivum xylanase inhibitor) [2] have been shown
to inhibit xylanases. XIP-I from wheat (Triticum aesti-
vum) represents the prototype of a novel class of
(b⁄a)
8
inhibitors that inhibits reversibly xylanases
belonging to glycoside hydrolase families (GHs) 10 and
11 (CAZY database http://afmb.cnrs-mrs.fr/CAZY/)
[3]. The structural features essential for xylanase inhibi-
tion were recently largely unravelled by the resolution
of the crystal structures of XIP-I in complex with a
GH10 xylanase from Aspergillus nidulans and a GH11
xylanase from Penicillium funiculosum [4]. The inhibi-
tion mechanism is novel since XIP-I possesses two inde-
pendent enzyme-binding sites, allowing binding to two
glycoside hydrolases with different folds [4].
XIP-I belongs to a large protein family (GH18) that
contains mostly chitinases and proteins of unknown
function. The crystal structure of XIP-I confirmed the
structural resemblance to GH18 chitinases [5]. In XIP-
I, however, clear structural differences in the region
corresponding to the active site of chitinases account
for its lack of enzymatic activity towards chitin [5–7].
XIP-type proteins were also isolated from rye,
durum wheat, barley and maize [8], but sequence infor-
mation is limited and the only clones available are
those encoding XIP-I (GenPept, AN: CAD19479) and
XIP-II (GenPept, AN: CAC87260), the other putative
XIP-type inhibitor from wheat (Triticum turgidum ssp.
Durum).
The widespread representation of XIP-type inhibi-
tors in cereals questions further the place ⁄evolution of
Keywords
chitinase; evolution; family 18 glycoside
hydrolase; proteinaceous xylanase inhibitors;
rice
*Present address
John Innes Centre, Norwich Research Park,
Colney, Norwich NR4 7UH, UK
(Received 16 December 2004, revised 3
February 2005, accepted 9 February 2005)
doi:10.1111/j.1742-4658.2005.04606.x
The xylanase inhibitor protein I (XIP-I), recently identified in wheat, inhib-
its xylanases belonging to glycoside hydrolase families 10 (GH10) and 11
(GH11). Sequence and structural similarities indicate that XIP-I is related
to chitinases of family GH18, despite its lack of enzymatic activity. Here
we report the identification and biochemical characterization of a XIP-type
inhibitor from rice. Despite its initial classification as a chitinase, the rice
inhibitor does not exhibit chitinolytic activity but shows specificities
towards fungal GH11 xylanases similar to that of its wheat counterpart.
This, together, with an analysis of approximately 150 plant members of
glycosidase family GH18 provides compelling evidence that xylanase inhibi-
tors are largely represented in this family, and that this novel function has
recently emerged based on a common scaffold. The plurifunctionality of
GH18 members has major implications for genomic annotations and pre-
dicted gene function. This study provides new information which will lead
to a better understanding of the biological significance of a number of
GH18 ‘inactivated’ chitinases.
Abbreviations
E:I
50
, molar ratio enzyme–inhibitor that gives 50% of inhibition; GH, glycoside hydrolase; pRIXI, putative rice xylanase inhibitor; RIXI, rice
xylanase inhibitor; rXIP-I, recombinant XIP-I produced in Pichia pastoris; SPR, surface plasmon resonance; XIP-I, xylanase inhibitor protein I;
XYNC, Penicillium funiculosum xylanase C.
FEBS Journal 272 (2005) 1745–1755 ª2005 FEBS 1745

this new class of protein within GH18. The existence
of several classes of GH18 chitinases in plants was pre-
viously suggested [9]. However the general impression
was that gene duplications, gene losses and perhaps
also translocations resulted in rather unreliable rela-
tionships for deriving evolutionary conclusions [10]. In
contrast to the abundant genetic information produced
from recent sequencing programmes of plant organ-
isms (rice and Arabidopsis), relatively little is known
about the enzymatic and structural properties of
GH18 plant chitinases. An emergent proportion
of sequences appear to encode plant inactivated chitin-
ases, such as narbonin and concanavalin B, the recep-
tor-like kinase Chrk1, and XIP [11]. Based on the
recent structural data obtained on XIP-I, can we ana-
lyze family GH18 and find other proteins with the
same function as XIP? This has implications for an
improved annotation of plant genes or ESTs and is
particularly important as there is no apparent relation-
ship between the old function (chitinase) and the newly
evolved one (xylanase inhibitor), despite sequence and
structural similarity.
No XIP-type protein was so far identified in rice.
Among the GH18 sequences isolated from the rice
genome [at least 23 – data from the Carbohydrate-
active enzymes database, http://afmb.cnrs-mrs.fr/
CAZY/ accessed 11 January 2005)], only two cDNA
sequences were shown to encode recombinant proteins
having chitinase activity [12] while others were classified
as putative rice class III chitinase(s) based on sequence
homology only [13]. In particular the (GenPept data-
bank; AN: BAA23810.1) clone shares higher similarity
with XIP-I than with ‘active’ chitinases and was thus
selected as a putative rice xylanase inhibitor (pRIXI).
In this work, we report for the first time the func-
tional identification of a rice ortholog of the wheat
XIP, originally classified as a rice class III chitinase
and analyze the features that allow discriminating the
subfamily of xylanase inhibitors within the large GH18
family.
Results
Production and structural characterization
of pRIXI
The pRIXI clone (GenPept databank; AN:
BAA23810.1) is expected to encode a protein of 304
residues with a predicted relative molecular mass of
33 946.8 and a theoretical pI of 9.33 [13]. In order to
address the functionality of this potential inhibitor, its
cDNA sequence and that of XIP-I were expressed in
conditions similar to those used for the production of
active basic chitinase in Pichia pastoris GS115 strain
[12], e.g. under the control of the alcohol oxidase pro-
moter and with an His-tag tail at the C-terminus. Both
recombinant XIP-I (rXIP-I) and pRIXI were produced
in P. pastoris with a high secretion yield of approxi-
mately 250 mgÆL
)1
. The recombinant proteins were
purified to apparent homogeneity from the culture
supernatant as a C-terminal tag fusion protein using
one step affinity chromatography. The purified pro-
teins migrated on SDS ⁄PAGE as a 33 and 37 kDa
single bands for pRIXI and rXIP-I, respectively. The
relative molecular mass of pRIXI, obtained by ESI-
MS, was 33 446 Da, thus in total agreement with the
predicted calculated mass including the myc epitope
and His-tag in C-terminal. In contrast, rXIP-I showed
an apparent relative molecular mass of 37 000 Da on
SDS ⁄PAGE, thus higher than the size of the native
protein from wheat (34 076 Da). Native XIP-I has
been reported to be weakly glycosylated and the two
N-glycosylation sites (Asn89 and Asn265) are occupied
[3,5,6]. These glycosylation sites are not present in the
rice homologue. The relative molecular mass discrep-
ancy between the native and recombinant proteins
may be explained by hyperglycosylation of the rXIP-I
in P. pastoris, as confirmed by mass spectrometry,
where five main peaks were identified (37 529, 37 692,
37 853, 37 873 and 38 015 Da). Isoelectric focusing
revealed that rXIP-I consisted of three molecular iso-
forms of pI 6.8–7.2–8.2 with a main band at pI 7.2.
This value differs from native XIP-I of pI 8.7–8.9 [6],
due to the insertion of the myc epitope and His-tag
in C-terminal. The recombinant pRIXI showed a pI
close to pH 9, thus in agreement with the calculated
pI of 8.7. The predominant N-terminal sequences,
EAEAEFAGGK for rXIP-I and EFGPAMAAGK for
pRIXI indicated that the two proteins were correctly
processed at the Kex2 and Ste13 signal cleavage sites,
respectively. Both recombinant proteins were recog-
nized by antibodies raised against His-tag. However,
although the rXIP-I was recognized by antibodies
raised against native XIP-I, there was no cross-reaction
with pRIXI (data not shown).
Functionality of the recombinant proteins
The recombinant proteins were tested for their chitinase
activity using two different size substrates. Using chitin
azure, a long and insoluble substrate, no chitinase activ-
ity could be detected at pH 5.5 and 8.0 for both pRIXI
and rXIP-I, confirming the lack of chitinase activity pre-
viously reported for native XIP-I at pH 5.5 in the same
conditions [6]. Interestingly, no activity could be detec-
ted using this substrate with the recombinant basic
A novel GH18 xylanase inhibitor identified in rice A. Durand et al.
1746 FEBS Journal 272 (2005) 1745–1755 ª2005 FEBS

chitinase (GenPept databank; AN: BAA22266.1) al-
though Streptomyces griseus chitinase, used as control,
was active. The activity of the proteins were then further
tested on a short and soluble substrate, 4-nitrophenyl
b-d-N,N¢,N¢¢-triacetylchitotriose [p-nitrophenol-(Glc-
NAc)
3
]. Using this substrate, the recombinant basic chi-
tinase showed a specific activity of 31.3 and 9.9 UÆmg
)1
at pH 5.5 and 8.0, respectively. However, neither pRIXI
nor rXIP-I showed any evidence for chitinase activity
even in a presence of a molar excess of inhibitors, 3.5 : 1
and 10 : 1 (inhibitor–basic chitinase) for pRIXI and
rXIP-I, respectively.
The specificity of pRIXI towards fungal and bacter-
ial GH10 and GH11 xylanases was compared to that
of rXIP-I (Table 1). The pattern of inhibition of rXIP-
I towards GH11 xylanases was similar to that previ-
ously reported for the native inhibitor (E : I
50
values,
Table 1). All the fungal GH11 xylanases were inhibited
by both pRIXI and rXIP-I up to a molar ratio E : I of
1 : 30 (Table 1), although the E : I
50
of pRIXI were
higher than those of rXIP-I. The lowest molar ratio
(1 : 6.5) was obtained for the Trichoderma longibrachi-
atum (M3) xylanase. Indeed, for the GH11 Aspergil-
lus niger xylanase the value of the E : I
50
is greater
than 1 : 52, in these conditions 34% of inhibition was
observed. As for native XIP-I, no inhibition was
observed for pRIXI and rXIP-I against two bacterial
GH11 xylanases from Bacillus subtilis and rumen
microorganism (M6) (Table 1). In contrast to both
native and recombinant XIP-I, none of the GH10 xy-
lanases from A. niger,A. aculeatus and A. nidulans
(fungal) or from Cellvibrio japonicus (bacterial) were
inhibited by pRIXI (Table 1).
Interaction of the inhibitors with xylanases
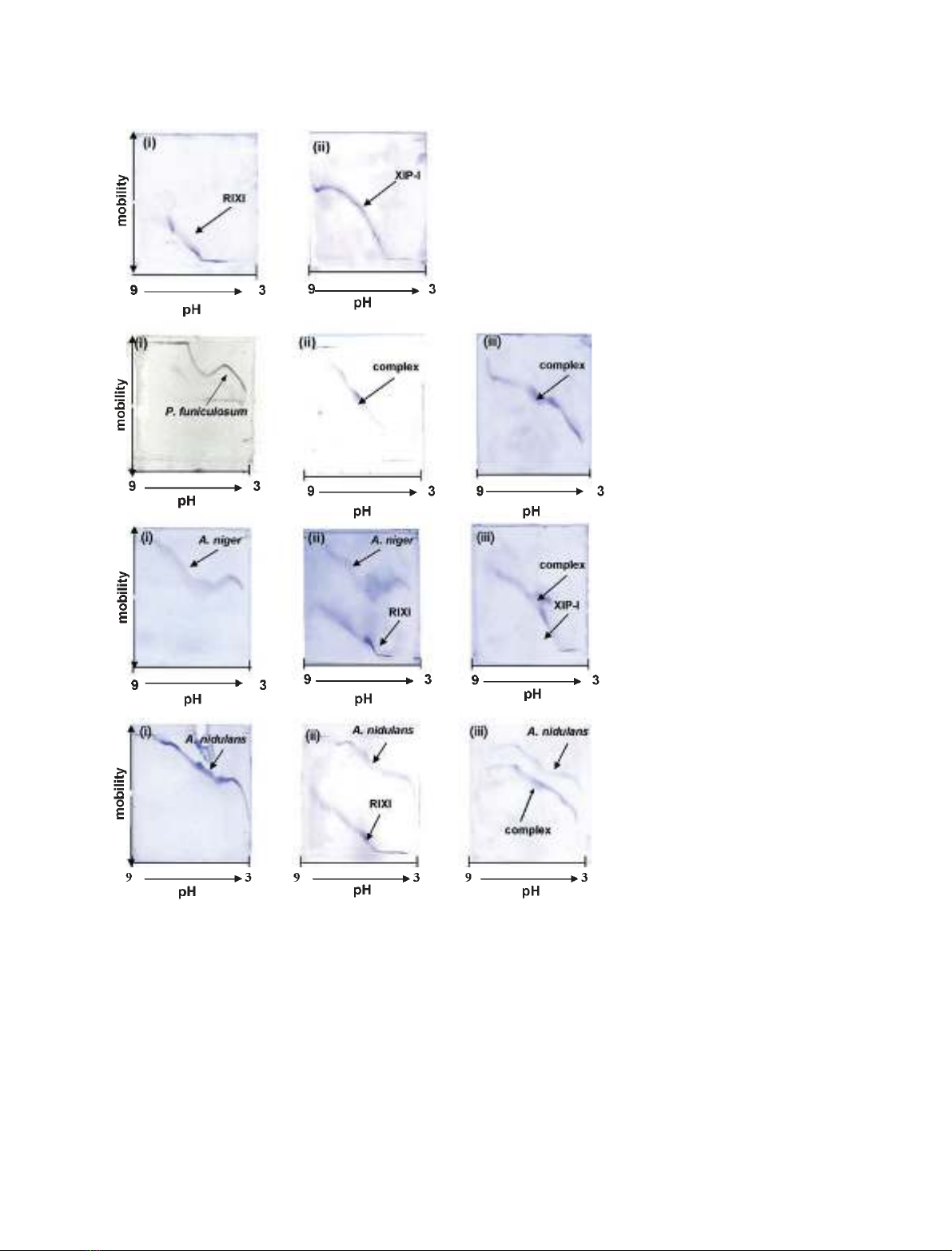
The relative affinities and pH dependencies of the inter-
action of XIP-I with xylanases were studied using titra-
tion curves. The GH11 XYNC from P. funiculosum
interacted with both pRIXI and rXIP-I across the
entire range of pH (Fig. 1B). However, although GH11
A. niger (Fig. 1C) and GH10 A. nidulans (Fig. 1D) xy-
lanases interacted with rXIP-I, no complex formation
was observed with pRIXI (Fig. 1C,D), in agreement
with the activity assays data, suggesting that pRIXI is
a weaker inhibitor than XIP-I. The interaction between
rXIP-I and A. niger xylanase occurred across a narrow
range of pH, as previously demonstrated with native
XIP-I [3]. In contrast, no complex was observed with
bacterial GH11 xylanases from rumen microorganism
M6 and B. subtilis (data not shown) in agreement with
the reported specificity of XIP-type inhibitors.
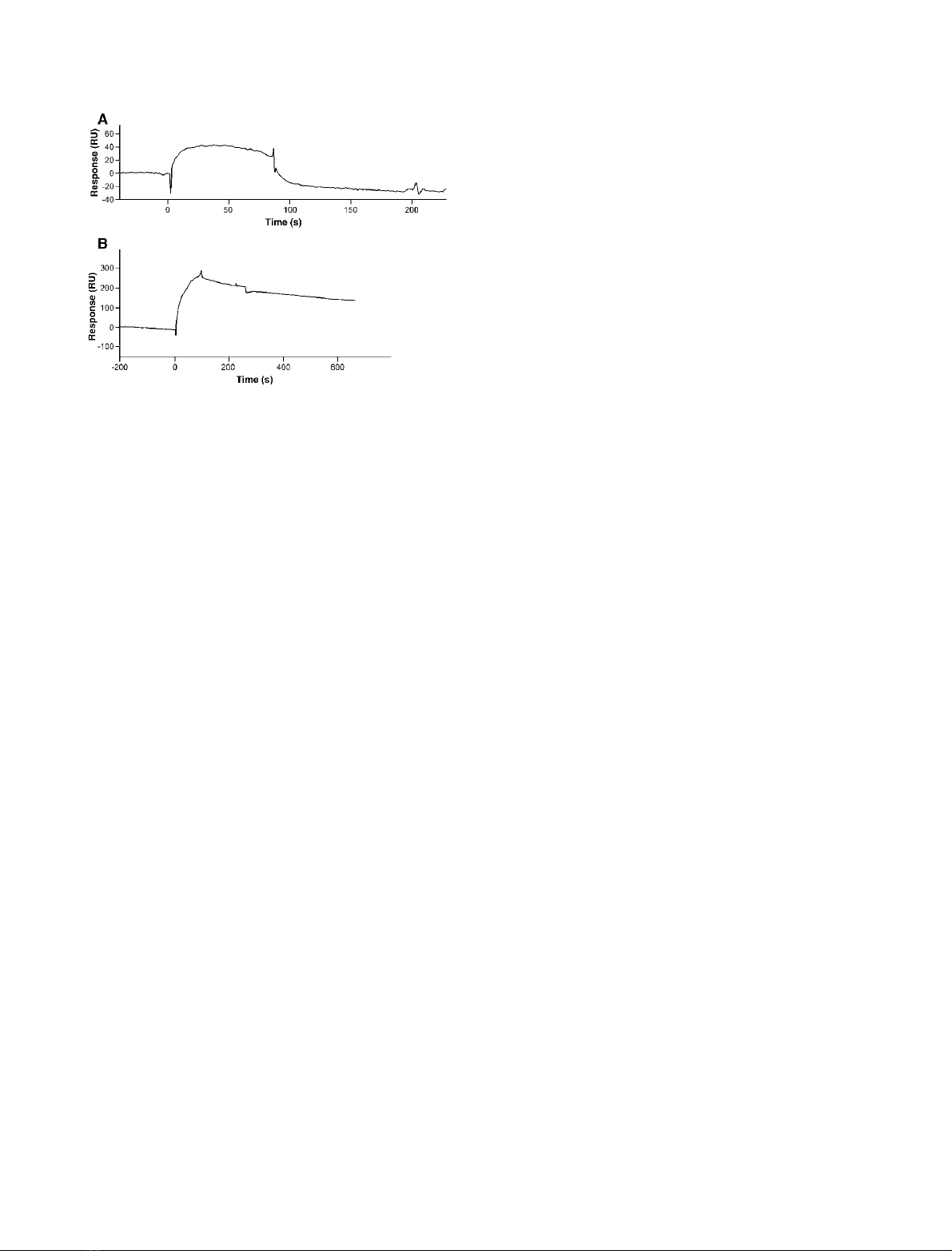
The molecular interaction between xylanases and
pRIXI or rXIP-I was further studied in real time by
using a biosensor based on surface SPR. The inhibitor
proteins were immobilized as a ligand on the dextran
surface of a chip whereas the P. funiculosum XYNC
xylanase was used as an analyte over the surface. The
sensorgrams for the interaction with XYNC are shown
in Fig. 2. The increase of RU from the baseline repre-
sents the binding of the xylanase to the surface-bound
inhibitor. The plateau line represents the steady-
state ⁄equilibrium phase of the XYNC–inhibitor inter-
action, whereas the decrease in RU from the plateau
represents the dissociation phase. The slow dissociation
phase observed on the SPR sensorgrams for the com-
plex between rXIP-I and XYNC suggests that the
interaction is stronger than the one previously reported
between native XIP-I and the A. niger xylanase [3], in
agreement with the inhibition constants reported for
these enzymes (K
i
¼3.4 nmand 317 nmfor XYNC
and A. niger xylanases, respectively) [3]. XYNC exhib-
ited a faster dissociation with pRIXI compared to
rXIP-I, in agreement with the lower value obtained
from E : I
50
for pRIXI (1 : 45 for pRIXI against
1 : 2.3 for rXIP-I and 1 : 1.6 for native XIP-I). SPR
analysis thus demonstrated that a faster dissociation
rate probably accounts for the weaker interaction
between pRIXI and XYNC compared to XIP-I.
Taken together, these data demonstrate that pRIXI
is not a chitinase but a novel XIP-type inhibitor in rice
and herein named RIXI for ‘RIce xylanase inhibitor’.
Table 1. Xylanase inhibition specificity of XIP-I and RIXI towards
xylanases.
RIXI XIP-I
Recombinant Recombinant Native
Family 11 xylanases
Fungal
A. niger Yes
a
1:3.6
b
1 : 2.1
XYNC P. funiculosum 1 : 45 1 : 2.3 1 : 1.6
T. longibrachiatum (M3) 1 : 6.5 1 : 2.3 1 : 1.1
Bacterial
B. subtilis No
c
No No
Rumen microorganism (M6) No No No
Family 10 xylanases
Fungal
A. nidulans No Yes 1 : 0.6
d
A. niger No Yes 1 : 0.7
A. aculeatus No No No
Bacterial
P. fluorescens No No No
a
Inhibition observed within the limit defined earlier (> 10% inhibi-
tion at E : I molar ratio up to 1 : 30 maximum [3]).
b
E:I
50
, molar
ratio of enzyme to inhibitor that gives 50% of inhibition.
c
No inhibi-
tion within the detection limit described in
a
.
d
From [3].
A. Durand et al. A novel GH18 xylanase inhibitor identified in rice
FEBS Journal 272 (2005) 1745–1755 ª2005 FEBS 1747
Discussion
RIXI is a novel xylanase inhibitor from rice
Our data clearly show that the rice putative chitinase
sequence (GenPept databank; AN: BAA23810.1) in
fact encodes a xylanase inhibitor. The previous lack
of detection of xylanase inhibitor in rice extracts can
be explained by the methodology used in the
previous reports [14,15]. Indeed the absence of detec-
tion by Western blotting is due to the lack of cross-
reactivity between purified RIXI and anti-XIP-I Igs
[14]. Furthermore, the weak interaction between
RIXI and GH11 A. niger xylanase explains why
affinity chromatography failed to interact with the
rice inhibitor [15] and why no xylanase inhibitor
activity was detected in rice extracts using the same
enzyme [14]. The observed weaker interaction is not
A
B
C
D
Fig. 1. Interaction of RIXI and rXIP-I with
xylanases. (A) Titration curves showing the
inhibitors. (i) RIXI; (ii) rXIP-I. (B) Titration cur-
ves showing the interaction between GH11
XYNC from P. funiculosum and the two rec-
ombinant inhibitors. (i) XYNC; (ii) a mixture
of XYNC and RIXI; (iii) a mixture of XYNC
and rXIP-I. (C) Titration curves showing the
interaction between GH11 A. niger xylanase
and the two inhibitors. (i) A. niger xylanase;
(ii) a mixture of A. niger xylanase and RIXI;
(iii) a mixture of A. niger xylanase and rXIP-I.
(D) Titration curves showing the interaction
between GH10 A. nidulans xylanase and the
two inhibitors. (i) A. nidulans xylanase; (ii) a
mixture of A. nidulans xylanase and RIXI; (iii)
a mixture of A. nidulans xylanase and rXIP-I.
For each experiment the molar ratio E : I
was identical (1 : 1) and 116 pmol of each
protein were loaded on the gel.
A novel GH18 xylanase inhibitor identified in rice A. Durand et al.
1748 FEBS Journal 272 (2005) 1745–1755 ª2005 FEBS
expected to be due to the lack of glycosylation of
RIXI, as glycosylation in XIP-I does not affect inhi-
bition specificity [4,5].
The presence of xylanase inhibitor in rice is not
surprising as hemicellulose in the cell walls of rice
cells is composed mainly of arabinoxylan [16] and the
ability to degrade xylan represents an important
attribute for a rice pathogen to infect plant tissues.
Indeed, secretion of xylanases by rice pathogens was
reported for Magnaporthe grisea, the fungal pathogen
that causes rice blast disease [17], and Xanthomonas
oryzae pv. oryzae, the causal agent of bacterial leaf
blight, a serious disease in rice [17–19]. The recent
demonstration that xylanases secreted by rice patho-
gens are important factors of their virulence agrees
with a potential role of RIXI in plant defence, as
proposed for XIP-I [20]. This hypothesis is reinforced
by the homology of RIXI with chitinases, which are
known to act in response to invading pathogens by
degrading polysaccharides of their cell wall. Class III
chitinases have been classified into pathogenesis-rela-
ted proteins (PR-8) because of their inducible expres-
sion upon infection by pathogens [21,22]. Plant
chitinases exhibit rapid evolution by acting as prime
targets for the coevolution of plant–pathogen interac-
tions. XIP-type proteins could have evolved from
chitinases as part of the plant defence pathway to act
both on the xylanases secreted by pathogens and on
the pathogen itself. In both cases, the function is ori-
entated towards a general role in plant defence and
the production of inhibitors prevents the plant to
undergo unnecessary metabolic costs.
XIP-type inhibitors represent a subfamily of GH18
GH18 includes chitinases from various species, inclu-
ding bacteria, fungi, nematodes, insects plants, and
mammals, but also a growing number of nonchitinase
proteins, the latter making genome and ESTs annota-
tions particularly unreliable (for instance RIXI was
thought to be a basic chitinase). Sequence-based famil-
ies such as those in CAZy, PFAM, etc., group together
proteins that have sometimes different functions. Here
the case is particularly tricky as the novel function has
been acquired relatively recently (in such a case, only
functional and structural characterization can help
building the necessary knowledge to enable prediction
methods). In the present work, novel biochemical and
structural information of XIP-type inhibitors are used
to test whether it is possible to better predict function-
ality within the GH18 family.
Although the overall sequence similarity between
GH18 chitinases is not particularly high (average pair-
wise 21%), their active site regions contain many
residues that are fully or highly conserved. The
most prominent motif dictating chitinase activity is
DxxDxDxE that includes the glutamate acting as the
catalytic acid. The GH18 members devoid of chitinase
or known enzymatic activity, all have nonconservative
substitutions of one of the acidic amino acid residues
in the catalytic region (Fig. 3). The XIP-type inhibitors
all have the third aspartic acid DxxDxDxE mutated
into an aromatic residue (Phe126
XIP-I
) whereas the cat-
alytic glutamate residue is only conserved in XIP-I
(Glu128
XIP-I
). The substitution of the critical Asp aci-
dic amino acid by a bulky residue thus is a major
determinant for the lack of chitinase activity reported
for XIP-I and RIXI. This suggests that another GH18
sequence (GenPept, AN: BAC10141.1) could be an
additional xylanase inhibitor in rice.
The inhibition specificity of RIXI can be explained
on the basis of the recently solved 3-D structure of
XIP-I in complex with a GH10 xylanase from A. nidu-
lans and a GH11 xylanase from P. funiculosum [4].
The inhibition of GH10 xylanase occurs through
extensive interactions between the two proteins. XIP-I
a7 helix (232–245) interacts with the loops forming the
xylanase groove; side chains emerging from the helix
point into the heart of the cleft and occupy the four
central subsites: )1 (Lys234
XIP-I
), +1 (Asn235
XIP-I
),
)2 (His232
XIP-I
), and +2 (Tyr238
XIP-I
), whereas
Lys246
XIP-I
sterically blocks access to subsite )3. Two
additional regions (loop b
6
a
6
from residue 193–205
and a8 helix 268–272) make contact with the enzyme.
These three regions are determinants for the inhibitory
activity. Although a7 and a8 helixes are pretty well
Fig. 2. SPR sensorgrams showing the interaction between XYNC ⁄
RIXI (A) and XYNC ⁄rXIP-I (B). In both panels, XYNC (14 lM)was
injected at a flow rate of 50 lLÆmin
)1
. The signal is indicated in
resonance units (RU) and time 0 corresponds to the injection of
XYNC.
A. Durand et al. A novel GH18 xylanase inhibitor identified in rice
FEBS Journal 272 (2005) 1745–1755 ª2005 FEBS 1749


























