
Role of electrostatics in the interaction between plastocyanin
and photosystem I of the cyanobacterium
Phormidium laminosum
Beatrix G. Schlarb-Ridley
1
, Jose
´A. Navarro
2
, Matthew Spencer
1
, Derek S. Bendall
1
, Manuel Herva
´s
2
,
Christopher J. Howe
1
and Miguel A. De la Rosa
2
1
Department of Biochemistry and Cambridge Centre for Molecular Recognition, University of Cambridge, UK;
2
Instituto de
Bioquı´mica Vegetal y Fotosı´ntesis, Centro de Investigaciones Cientı´ficas Isla de la Cartuja, Universidad de Sevilla y CSIC, Spain
The interactions between photosystem I and five charge
mutants of plastocyanin from the cyanobacterium Phormi-
dium laminosum were investigated in vitro. The dependence
of the overall rate constant of reaction, k
2
, on ionic strength
was investigated using laser flash photolysis. The rate con-
stant of the wild-type reaction increased with ionic strength,
indicating repulsion between the reaction partners. Remov-
ing a negative charge on plastocyanin (D44A) accelerated the
reaction and made it independent of ionic strength; removing
a positive charge adjacent to D44 (K53A) had little effect.
Neutralizing and inverting the charge on R93 slowed the
reaction down and increased the repulsion. Specific effects of
MgCl
2
were observed for mutants K53A, R93Q and R93E.
Thermodynamic analysis of the transition state revealed
positive activation entropies, suggesting partial desolvation
of the interface in the transition state. In comparison with
plants, plastocyanin and photosystem I of Phormidium
laminosum react slowly at low ionic strength, whereas the two
systems have similar rates in the range of physiological salt
concentrations. We conclude that in P. laminosum, in con-
trast with plants in vitro, hydrophobic interactions are more
important than electrostatics for the reactions of plastocya-
nin, both with photosystem I (this paper) and with cyto-
chrome f[Schlarb-Ridley, B.G., Bendall, D.S. & Howe, C.J.
(2002) Biochemistry 41, 3279–3285]. We discuss the impli-
cations of this conclusion for the divergent evolution of
cyanobacterial and plant plastocyanins.
Keywords: cyanobacteria; electron transfer; photosystem I;
plastocyanin; weak interaction.
Electron-transfer chains like that of oxygenic photosyn-
thesis impose special restraints on the proteins involved.
Reactions must be fast to allow rapid turnover of the
chain. Binding between the reaction partners must be
transient, while at the same time sufficient specificity needs
to be retained. Surface properties of proteinaceous reac-
tion partners play a crucial role in meeting these criteria.
The aim of our research was to increase our understand-
ing of how one property of the protein surface, the charge
pattern, influences the rate constant of the overall reaction
and how it may have evolved. Our model protein is
plastocyanin, a soluble photosynthetic redox protein
which accepts an electron from cytochrome fin the
cytochrome bf complex and passes it on to P
700
+
in
photosystem I. In a previous study [1], we mutated
negatively and positively charged residues on the proposed
interaction site of plastocyanin with cytochrome fand
analysed the reaction of these mutants with the soluble
redox-active domain of cytochrome f(Cyt f) in vitro.This
paper presents results on the interaction in vitro between a
representative subset of these charge mutants with the
physiological electron acceptor of plastocyanin, photosys-
tem I. Hence, we can compare two sets of protein–protein
interactive surfaces operating in the same compartment
with similar functional selection pressures, with the aim of
identifying common features.
The organism from which plastocyanin and both its
reaction partners, Cyt f [1] and photosystem I (this paper),
were taken is a moderately thermophilic cyanobacterium,
Phormidium laminosum. Studying these photosynthetic
electron-transfer reactions of cyanobacteria is of evolu-
tionary interest: whereas the overall three-dimensional
structure of plastocyanin is highly conserved among plants
and cyanobacteria, the surface charge pattern varies
greatly [1]. Comparing cyanobacterial data with the wealth
of information available for the higher plant reaction [2–5]
reveals which functional aspects are variable. Further-
more, the type I copper protein plastocyanin can be
replaced by cytochrome c
6
, a redox protein of similar size
but entirely different folding, in a number of eukaryotic
algae and cyanobacteria including P. laminosum [6,7].
Hence two more sets of protein–protein interactive
surfaces with the same function as Cyt f – plastocyanin
and plastocyanin–photosystem I – are available for identi-
fication of features common to interprotein electron-
transfer reactions [4,7]. To our knowledge, this is the first
Correspondence to B. G. Schlarb-Ridley, Department of Biochemistry,
University of Cambridge, Building O, The Downing Site,
Cambridge CB2 1QW, UK.
Fax: + 44 1223 333345, Tel.: + 44 1223 333684,
E-mail: bgs9@mole.bio.cam.ac.uk
Abbreviations: Cyt f, soluble redox-active domain of cytochrome f;
k
obs
, observed first-order rate constant; k
on
, rate constant of protein
association; k
off
, rate constant of complex dissociation before electron
transfer has taken place; k
et
, rate constant of intracomplex electron
transfer; k
2
, bimolecular rate constant of the overall reaction; k
¥
,k
2
at
infinite ionic strength.
(Received 10 June 2002, revised 5 September 2002,
accepted 15 October 2002)
Eur. J. Biochem. 269, 5893–5902 (2002) FEBS 2002 doi:10.1046/j.1432-1033.2002.03314.x

case in which kinetic data of the interaction of plastocya-
nin with both Cyt f and photosystem I have been
collected in a homologous cyanobacterial system. This is
essential for informed discussion of evolutionary relation-
ships.
The structure and charge properties of plastocyanin have
been described previously in detail (Introduction in [1]). Its
primary electron acceptor is P
+
700
of photosystem I, a
photo-oxidized chlorophyll a-dimer. The crystal structure
of a cyanobacterial photosystem I has been solved at a
resolution of 2.5 A
˚[8]. In higher plants, the positively
charged N-terminal lumenal helix of PsaF has been shown
to be involved in binding of plastocyanin [9,10]. In
cyanobacteria, deletion of PsaF did not change the kinetics
of photosystem I reduction by either plastocyanin or
cytochrome c
6
[10,11]. Schubert et al. [12] suggest that, in
cyanobacteria, subunits PsaA and PsaB are largely respon-
sible for binding plastocyanin or cytochrome c
6
in a shallow
pocket.
In the reaction between photosystem I and plastocya-
nin from different organisms, three different types of
kinetics have been observed, which may represent vari-
ations on a single reaction scheme [13,14]. Type I kinetics
are characterized by monophasic decay of the absorbance
of photo-oxidized P
+
700
at 820 nm on reduction by
plastocyanin, and linear dependence of the observed
pseudo-first-order rate constant k
obs
on the plastocyanin
concentration. This type is observed for weak interac-
tions: in a range of experimentally reasonable plastocy-
anin concentrations, no sign of saturation is apparent.
Type II also exhibits monophasic kinetics; however, k
obs
approaches a saturating value at high plastocyanin
concentrations, which provides explicit evidence for
complex formation followed by intracomplex electron
transfer. Type III shows biphasic kinetics, which provides
evidence for the formation of an additional reaction
complex (compared to Type II) so that rearrangement
must occur before intracomplex electron transfer. The
reaction between plastocyanin and photosystem I of
P. laminosum is of Type I [7].
Determination of the ionic strength dependence of rates is
an important method of studying electrostatic interactions
[1]. The salt commonly added to increase ionic strength is
NaCl. However, it has been reported that bivalent cations
can play a specific role in the reaction in vitro between
photosystem I and both plastocyanin [15–17] and cyto-
chrome c
6
[13,18–21] by forming electrostatic bridges
between negative charges on the interacting surfaces. In
this study, we investigated the dependence of the second-
order rate constant of the overall reaction, k
2
, on both NaCl
and MgCl
2
concentration.
Information about the thermodynamic parameters of
the transition state can be obtained by measuring the
temperature dependence of k
2
. This analysis has been
performed for the interactions of plastocyanin and/or
cytochrome c
6
with their respective homologous photo-
system I from various plants, green algae and cyanobac-
teria [14,15,19,22] (including P. laminosum wild-type [7]).
We determined the activation parameters and their
dependence on NaCl and MgCl
2
concentration for the
reaction of P. laminosum photosystem I with P. lamino-
sum plastocyanin wild-type as well as five charge
mutants.
MATERIALS AND METHODS
Molecular biology and mutagenesis
Molecular biological methods were essentially as described
by Schlarb-Ridley et al.[1].
Protein methods
Expression, purification and characterization of wild-type
and mutant plastocyanins were carried out essentially as in
Schlarb et al. [23].
Photosystem I preparations
P. laminosum photosystem I particles were obtained by
solubilization with b-dodecyl maltoside as described by
Ro
¨gner et al. [24] and Herva
´set al. [21]. The chlorophyll/
P
700
ratio of the resulting photosystem I preparation was
150 : 1. The P
700
content in photosystem I samples was
calculated from the photoinduced absorbance increase at
820 nm using an absorption coefficient of 6.5 m
M
)1
Æcm
)1
[25]. Chlorophyll concentration was determined by the
method of Arnon [26].
Kinetic analysis
The second-order rate constant, k
2
, and its ionic strength
dependence were measured using laser-flash-induced
absorbance changes of photosystem I at 820 nm. Unless
stated otherwise, the experimental setup and programmes
used in the analysis were as in Herva
´set al.[13].The
standard experimental conditions were as described by
De la Cerda et al. [27]. Measurements of the dependence of
k
obs
on the concentration of plastocyanin were carried out in
the following buffer: 20 m
M
tricine/KOH (pH 7.5), 10 m
M
MgCl
2
, 100 l
M
methyl viologen and 0.03% (w/v) b-dodecyl
maltoside to which photosystem I-enriched particles
(0.39 mg chlorophyll per ml) were added. The same reaction
mixture but without the 10 m
M
MgCl
2
was used for
measuring the dependence of k
2
on ionic strength. The ionic
strength was adjusted with small aliquots of concentrated
solutions of NaCl or MgCl
2
, and correction was made for
the resulting dilution of the reaction mixture. All experi-
ments were carried out at 278, 283, 288, 293 and 298 K.
Thermodynamic activation parameters DH
,DS
and DG
were obtained according to the transition state theory by
fitting plots of k
2
/Tvs. Tto the Eyring equation:
k2
T¼kB
hexpðDGz=RTÞ
¼kB
hexpðDHz=RTÞexpðDSz=RÞð1Þ
where k
B
is the Boltzmann constant, his the Planck constant,
and Ris the gas constant. Nonlinear regression by the least-
squares method gave the standard error of DG
. To obtain an
independent error estimate for each of the correlated
parameters DH
and DS
, the Exhaustive Search Method
[28,29] was applied. Plots of rate constants, k
2
, against ionic
strength were fitted to the monopole–monopole version of
the Watkins equation (Eqn 2) by a nonlinear least-squares
method (
KALEIDAGRAPH
TM version 3.51; Synergy Software):
5894 B. G. Schlarb-Ridley et al.(Eur. J. Biochem. 269)FEBS 2002
k2¼k1exp½Vii expð0:3295q
ffiffi
I
pÞ=ð1þ0:3295q
ffiffi
I
pÞ
ð2Þ
where qis the radius of the interactive site (in A
˚), and the
factor 0.3295
ffiffi
I
pis the Debye-Hu
¨ckel parameter jat 298 K
[30]. The allowable error was set to 10
)4
%. For the criteria
used to determine the data range, see the Discussion.
Overall errors in the experimental determination of kinetic
constants were estimated to be 10%.
Electrostatic potentials
Electrostatic potentials of wild-type and mutant plastocy-
anins in the reduced form were calculated by a finite
difference solution of the linear Poisson–Boltzmann equa-
tion with
DELPHI
II [31]. The
SWISS
-
PDBVIEWER
was used to
add polar and aromatic ring hydrogens to chain A of pdb
file 1baw, and was also used to introduce mutations. Atomic
radii and partial charges were assigned from the PARSE list
of Sitkoff et al. [32].
RESULTS
Concentration dependence of
k
obs
and standard thermodynamic analysis
Five charge mutants of plastocyanin from P. laminosum
were chosen for analysis with wild-type photosystem I
isolated from the same organism (Fig. 1). All of them were
in a surface patch shown to interact with photosystem I in
the plant case [33]. One mutant neutralized a negative
charge (D44A), one neutralized an adjacent positive charge
(K53A), and three neutralized or inverted the charge on R93
(R93A, R93Q, R93E), a residue situated close to the charge
cluster that includes D44 and K53 and at the edge of the
hydrophobic flat end of the protein surrounding the copper
ligand H92. R93 has been shown to be essential for the
interaction of plastocyanin with photosystem I in Anabaena
[15], and is highly conserved in cyanobacterial plastocya-
nins. Mutagenesis, expression, purification and character-
ization of the plastocyanins has been described [1].
Representations of the electrostatic surfaces showing the
changes introduced by the mutations are displayed in Fig. 1.
The decay of the flash-induced absorbance of P
700
+
at
820 nm was monoexponential for all proteins at each of the
five temperatures (278, 283, 288, 293 and 298 K). In the
range of concentrations and temperatures used in this study,
k
obs
showed no sign of rate saturation. The best interpret-
ation of the results as a whole was a linear response to
plastocyanin concentration through the origin. Examples at
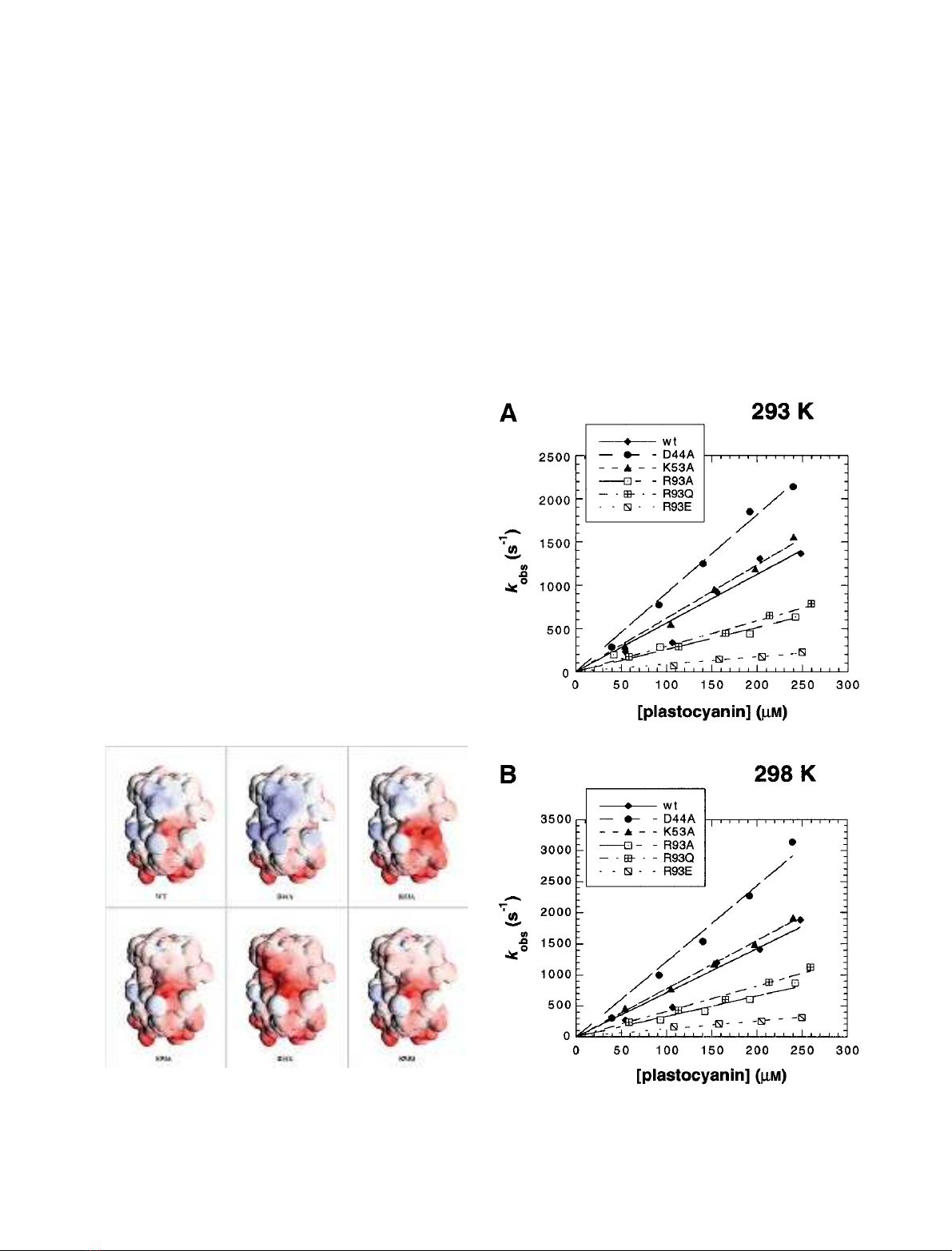
293 K and 298 K are shown in Fig. 2. Thus wild-type and
Fig. 1. Representations of the electrostatic surface potentials of wild-
type and mutant P. laminosum plastocyanin drawn with GRASP [50].
The molecular surface (probe radius 1.4 A
˚) is coloured according to
electrostatic potential on a scale of red (acidic) to blue (basic). The
orientation is similar to that of Fig. 2 of [1].
Fig. 2. Dependence of k
obs
on plastocyanin concentration: wild-type and
mutant P. laminosum plastocyanin reacting with wild-type P. laminosum
photosystem I at (A) 293 K and (B) 298 K. The data were fitted to the
equation k
obs
¼k
2
[plastocyanin].
FEBS 2002 Electrostatics in electron transfer: Pc–PSI (Eur. J. Biochem. 269) 5895
all mutants were treated as following kinetic Type I. Balme
et al. [7] have already reported Type I behaviour for the
wild-type protein. From the slopes of the linear regressions
in Fig. 2A the bimolecular rate constants for the overall
reaction, k
2
, were determined (Table 1). The rate constant
increased when a negatively charged residue was neutralized
(D44A), hardly changed when an adjacent positively
charged residue was neutralized (K53A), but decreased
markedly when the charge of R93 was neutralized (R93A,
R93Q), and even more so when it was inverted (R93E). The
results are summarized in Table 1 and are qualitatively
similar to those obtained in the reaction with Cyt f [1].
Balme et al. [7] have previously reported a slightly higher
value for k
2
of the wild-type reaction, and we attribute this
to the use of different photosystem I preparations.
The thermodynamic parameters obtained from tempera-
ture-dependence measurements of k
obs
at 10 m
M
MgCl
2
show that DG
decreases slightly for D44A compared with
wild-type, remains essentially unchanged for K53A, and
increases for all three R93 mutants, most markedly for
R93E (Table 1). Owing to the correlation between DH
and
DS
, their independent errors, determined by the Exhaustive
Search Method, are large. Hence in all but one case (DH
of
R93E), DS
and DH
lie within the 67% confidence interval
of the wild-type values. However, the trends parallel those
seen for DG
: a decrease relative to wild-type for D44A, no
change for K53A, and an increase for all three R93 mutants,
again most pronounced in R93E. It is noteworthy that, with
67% confidence, all DS
values are positive under these
conditions. Implications for the structure of the transition
state are described in the Discussion.
Ionic strength dependence
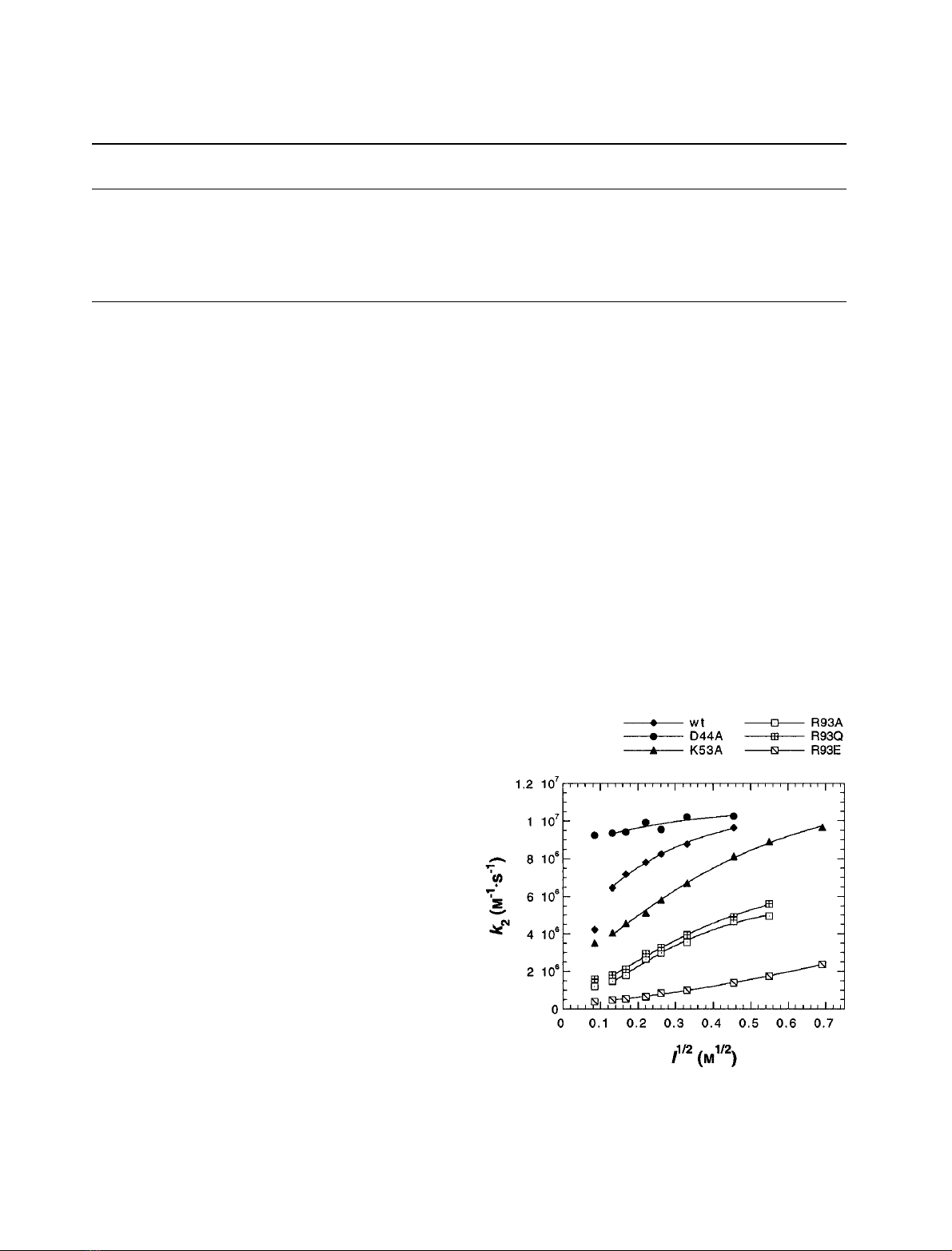
Response to NaCl. The dependence of the second-order
rate constant, k
2
, on the concentration of NaCl was
investigated at five different temperatures (278, 283, 288,
293 and 298 K). Figure 3 shows the result for all proteins at
298 K; the other temperatures gave analogous results. For
wild-type plastocyanin, the rate increased with increasing
salt concentration, as observed by Balme et al.[7].Thisisin
clear contrast with the reaction of wild-type plastocyanin
with Cyt f, where the rate decreases with increasing ionic
strength [1]. The mutant D44A showed no dependence on
ionic strength, but K53A reacted slightly more slowly than
wild-type and exhibited a shallower dependence on NaCl
concentration. R93A and R93Q were slower still with a
similar steepness, and again R93E showed the most
pronounced effect. Experimental results were fitted to the
Watkins equation (see Materials and methods), as shown in
Fig. 3, to obtain estimates of k
2
at infinite ionic strength (k
¥
)
(Table 1). Modification of charge at positions 44 and 53 had
no significant effect on k
¥
, but values were significantly
lower for mutants of R93.
Response to MgCl
2
.In some systems, enhancement effects
have been reported when bivalent rather than univalent
cations were used in measurements of ionic strength
dependence (see the Introduction). Hence, the dependence
of k
2
of wild-type and all mutants on the concentration of
MgCl
2
was investigated at 278, 283, 288, 293 and 298 K.
Table 1. Kinetic and thermodynamic parameters of the reaction between wild-type and mutant P. laminosum plastocyanin with wild-type P. laminosum
photosystem I. Errors given are either standard errors obtained from curve fitting by least squares (k
2
,k
¥
,DG
) or 67% confidence limits derived by
the Exhaustive Search Method (DH
,DS
).
Plastocyanin
k
2
at 298 K
a
(l
M
)1
Æs
)1
)
k
¥
at 298 K
b
(l
M
)1
Æs
)1
)
k
¥
at 298K
c
(l
M
)1
Æs
)1
)
DG
a
(kJÆmol
)1
)
DH
a
(kJÆmol
)1
)
DS
a
(JÆmol
)1
ÆK
)1
)
Wild-type 7.1 ± 0.5 10.6 ± 0.5 10.0 ± 0.7 34.06 ± 0.08 40.2 (34.2–46.5) 20.8 (0.3–42.5)
D44A 12.1 ± 0.5 10.9 ± 2.1 11.7 ± 0.2 32.66 ± 0.06 37.9 (34.8–41.1) 17.9 (7.3–28.8)
K53A 7.8 ± 0.1 12.4 ± 0.7 12.3 ± 1.0 33.74 ± 0.08 39.8 (34.5–45.4) 20.7 (2.5–39.8)
R93A 3.3 ± 0.2 5.9 ± 0.5 7.6 ± 1.3 36.00 ± 0.12 47.5 (42.7–52.5) 39.2 (22.9–56.4)
R93Q 4.1 ± 0.1 7.0 ± 0.6 6.7 ± 0.5 35.45 ± 0.11 46.2 (44.4–48.0) 36.6 (30.5–42.9)
R93E 1.3 ± 0.1 8.5 ± 3.4 3.4 ± 0.6 38.37 ± 0.12 50.4 (47.9–52.9) 40.9 (32.4–49.6)
a
Buffer used contained 10 m
M
MgCl
2
.
b
Buffer contained no MgCl
2
; ionic strength was adjusted with NaCl. The first datapoint was not
included in the fit (see Discussion).
c
Buffer contained no NaCl; ionic strength was adjusted with MgCl
2
. The first datapoint was not included
in the fit (see Discussion).
Fig. 3. Ionic strength dependence (NaCl) of k
2
: wild-type and mutant
P. laminosum plastocyanin reacting with wild-type P. laminosum photo-
system I at 298 K. All measured data points are shown; for the fits to
the Watkins equation the first data point was excluded (see Discus-
sion). Values for k
¥
obtained from the fit are given in Table 1.
5896 B. G. Schlarb-Ridley et al.(Eur. J. Biochem. 269)FEBS 2002
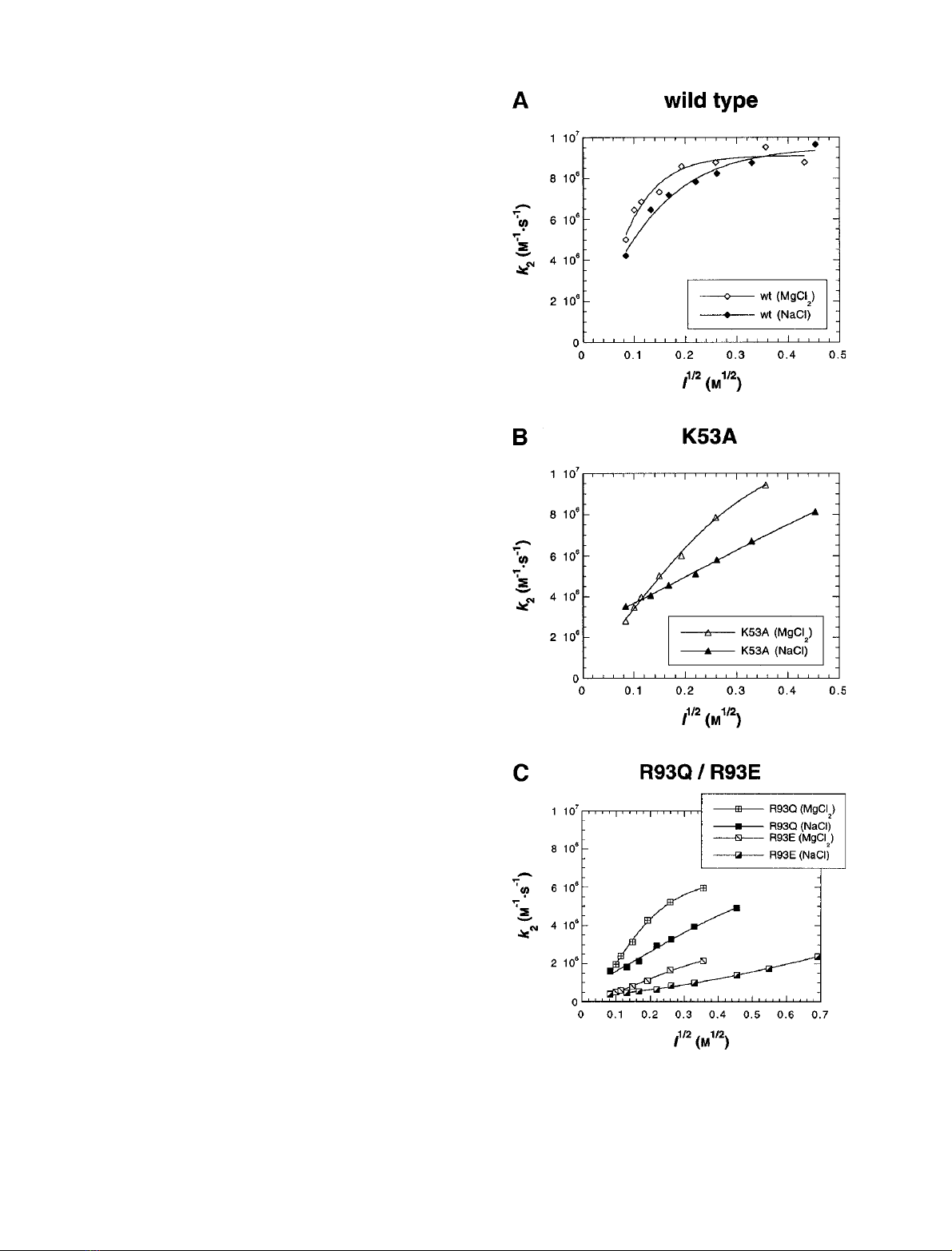
Wild-type plastocyanin showed little or no significant
difference in its ionic strength dependence whether NaCl
or MgCl
2
was used (Fig. 4A; this has also been reported in
[7]). The same was the case for the mutants D44A and
R93A. For mutants K53A, R93Q and R93E, however, the
rate constant increased faster with ionic strength when
MgCl
2
rather than NaCl was added (Fig. 4B,C). Figure 4
shows the results obtained at 298 K, and analogous effects
were observed at the other temperatures.
Activation parameters
Nonlinear Eyring plots of the effect of temperature on k
2
at
each salt concentration were used to determine the effect of
ionic strength on the activation enthalpy, entropy and free
energy. No significant difference was observed between the
thermodynamics of the NaCl and MgCl
2
dependencies.
Figure 5A–C shows DH
and –TDS
at 298 K plotted
against the square root of ionic strength (using MgCl
2
)for
wild-type, K53A and R93E. The noise in the wild-type data
buries any trend, if there is one. Although there is still
considerable noise in the K53A data, a trend in both DH
(increasing with ionic strength) and –TDS
(decreasing with
increasing ionic strength) is emerging. For R93E, this trend
is clear and considerably larger than any noise. These trends
have also been observed for DH
and –TDS
of plastocyanin
and cytochrome c
6
from Synechocystis sp. PCC 6803, and a
trend of opposite sign has been reported for plastocyanin
from Anabaena (each reacting with their respective homo-
logous photosystem I), whereas Anabaena sp. PCC 7119
cytochrome c
6
showed an increase for both DH
and –TDS
[14].
Comparison between
P. laminosum
and spinach
The response to ionic strength of the reaction between
P. laminosum plastocyanin and photosystem I was in
marked contrast with the behaviour of the homologous
system in spinach. A direct comparison of the two systems
at 298 K is shown in Fig. 6 (spinach data taken from [14]).
Below 100 m
M
NaCl, the plant system reacted at least one
order of magnitude faster than that of the cyanobacterium,
but with increasing NaCl concentration the difference
diminished; the point of intersection of the two curves can
be extrapolated to 270 m
M
NaCl. Eyring plots can be
used to extrapolate k
2
to 318 K [7], the temperature at
which P. laminosum is cultured. When the resulting data
were plotted together with the spinach data at 298 K (an
acceptable growth temperature for spinach), the point of
intersection moved to 150 m
M
NaCl. To our knowledge,
the ionic strength of the thylakoid lumen has not been
determined. Published values of the ionic strength in the
stroma of chloroplasts vary from 130 m
M
to 200 m
M
[34,35], and it seems reasonable to assume that the lumenal
ionic strength lies within a similar range. Hence, at
physiological ion concentrations and temperatures, the
plant and cyanobacterial systems show similar rates.
DISCUSSION
To our knowledge, the work described here and in the
related publications [1,7] is the first kinetic analysis of the
in vitro interactions Cyt f–plastocyanin and plastocyanin–
Fig. 4. Comparison of ionic strength curves obtained by using NaCl or
MgCl
2
: wild-type and mutant P. laminosum plastocyanin reacting with
wild-type P. laminosum photosystem I at 298 K. (A) wild-type; (B)
K53A; (C) R93Q and R93E.
FEBS 2002 Electrostatics in electron transfer: Pc–PSI (Eur. J. Biochem. 269) 5897



















![Bộ Thí Nghiệm Vi Điều Khiển: Nghiên Cứu và Ứng Dụng [A-Z]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/10301767836127.jpg)
![Nghiên Cứu TikTok: Tác Động và Hành Vi Giới Trẻ [Mới Nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250429/kexauxi8/135x160/24371767836128.jpg)





