
Knock-out of the chloroplast-encoded PSI-J subunit
of photosystem I in Nicotiana tabacum
PSI-J is required for efficient electron transfer and stable
accumulation of photosystem I
Andreas Hansson
1
, Katrin Amann
2
, Agnieszka Zygadlo
1
,Jo
¨rg Meurer
2
, Henrik V. Scheller
1
and Poul E. Jensen
1
1 Plant Biochemistry Laboratory, Department of Plant Biology, Faculty of Life Sciences, University of Copenhagen, Frederiksberg, Denmark
2 Department Biologie I, Botanik, Ludwig-Maximilians-Universita
¨t-Mu
¨nchen, Germany
The photosystem I (PSI) complex of higher plants con-
sists of at least 19 different polypeptides [1–3]. PSI
mediates light-driven electron transfer from reduced
plastocyanin (Pc) in the thylakoid lumen to oxidized
ferredoxin in the stroma. The PSI core in higher plants
contains at least 15 different subunits named PSI-A to
PSI-L, PSI-N to PSI-P. Two subunits present in
cyanobacteria, PSI-M and PSI-X, are missing from
plants. In addition to the PSI core, higher plants con-
tain a peripheral antenna associated with PSI, also
known as light-harvesting complex I (LHCI), which is
mainly composed of four different Lhca proteins.
The major subunits of PSI, PSI-A and PSI-B, form a
heterodimer, which binds the components of the elec-
tron-transfer chain: the primary electron donor P700
and the electron acceptors A
0
,A
1
and F
x
[1,4,5]. The
two remaining electron acceptors, F
A
and F
B
, are bound
to the PSI-C subunit. PSI-C is located towards the stro-
mal side of PSI and, together with PSI-D and PSI-E,
provides the docking side for soluble ferredoxin [5,6].
Keywords
antenna size; electron transport;
photosynthesis; plastocyanin kinetics;
thylakoid membrane
Correspondence
P. E. Jensen, Plant Biochemistry Laboratory,
Department of Plant Biology, Faculty of Life
Sciences, University of Copenhagen, 40
Thorvaldsensvej, DK-1871 Frederiksberg C,
Denmark
Fax: +45 35 28 33 33
Tel: +45 35 28 33 40
E-mail: peje@life.ku.dk
(Received 30 August 2006, revised 21
December 2006, accepted 31 January 2007)
doi:10.1111/j.1742-4658.2007.05722.x
The plastid-encoded psaJ gene encodes a hydrophobic low-molecular-mass
subunit of photosystem I (PSI) containing one transmembrane helix. Ho-
moplastomic transformants with an inactivated psaJ gene were devoid of
PSI-J protein. The mutant plants were slightly smaller and paler than wild-
type because of a 13% reduction in chlorophyll content per leaf area
caused by an 20% reduction in PSI. The amount of the peripheral
antenna proteins, Lhca2 and Lhca3, was decreased to the same level as the
core subunits, but Lhca1 and Lhca4 were present in relative excess. The
functional size of the PSI antenna was not affected, suggesting that PSI-J
is not involved in binding of light-harvesting complex I. The specific PSI
activity, measured as NADP
+
photoreduction in vitro, revealed a 55%
reduction in electron transport through PSI in the mutant. No significant
difference in the second-order rate constant for electron transfer from
reduced plastocyanin to oxidized P700 was observed in the absence of PSI-
J. Instead, a large fraction of PSI was found to be inactive. Immunoblot-
ting analysis revealed a secondary loss of the luminal PSI-N subunit in PSI
particles devoid of PSI-J. Presumably PSI-J affects the conformation of
PSI-F, which in turn affects the binding of PSI-N. This together renders a
fraction of the PSI particles inactive. Thus, PSI-J is an important subunit
that, together with PSI-F and PSI-N, is required for formation of the plast-
ocyanin-binding domain of PSI. PSI-J is furthermore important for stabil-
ity or assembly of the PSI complex.
Abbreviations
Chl, chlorophyll; Cyt, cytochrome; LHC, light-harvesting complex; Pc, plastocyanin; PS, photosystem.
1734 FEBS Journal 274 (2007) 1734–1746 ª2007 The Authors Journal compilation ª2007 FEBS
In plants, the three low-molecular-mass subunits,
PSI-F, PSI-G and PSI-N, have been implicated in the
interaction between PSI and Pc [7–9]. PSI-F contains
one transmembrane helix and is exposed to both the
lumen and the stroma: its rather large N-terminal
domain is situated in the lumen [10], whereas the
C-terminus is in contact with PSI-E on the stromal
side [6]. The N-terminal part of PSI-F and luminal
interhelical loops of PSI-A and PSI-B form a docking
site for Pc or cytochrome (Cyt) c
6
[11–15]. In plants,
which only use Pc as an electron donor to PSI, a
longer N-terminal domain contributes to a helix–
loop–helix motif [10], which specifically enables more
efficient Pc binding and, as a result, two orders of
magnitude faster electron transfer from Pc to P700
[16]. PSI-N is unique to eukaryotic PSI and is entirely
located in the thylakoid lumen. However, the recently
published structural model of higher-plant PSI based
on a crystal structure at 4.4 A
˚does not reveal the pres-
ence of PSI-N [10], and cross-linking experiments have
shown little interaction between PSI-N and other small
PSI subunits [17].
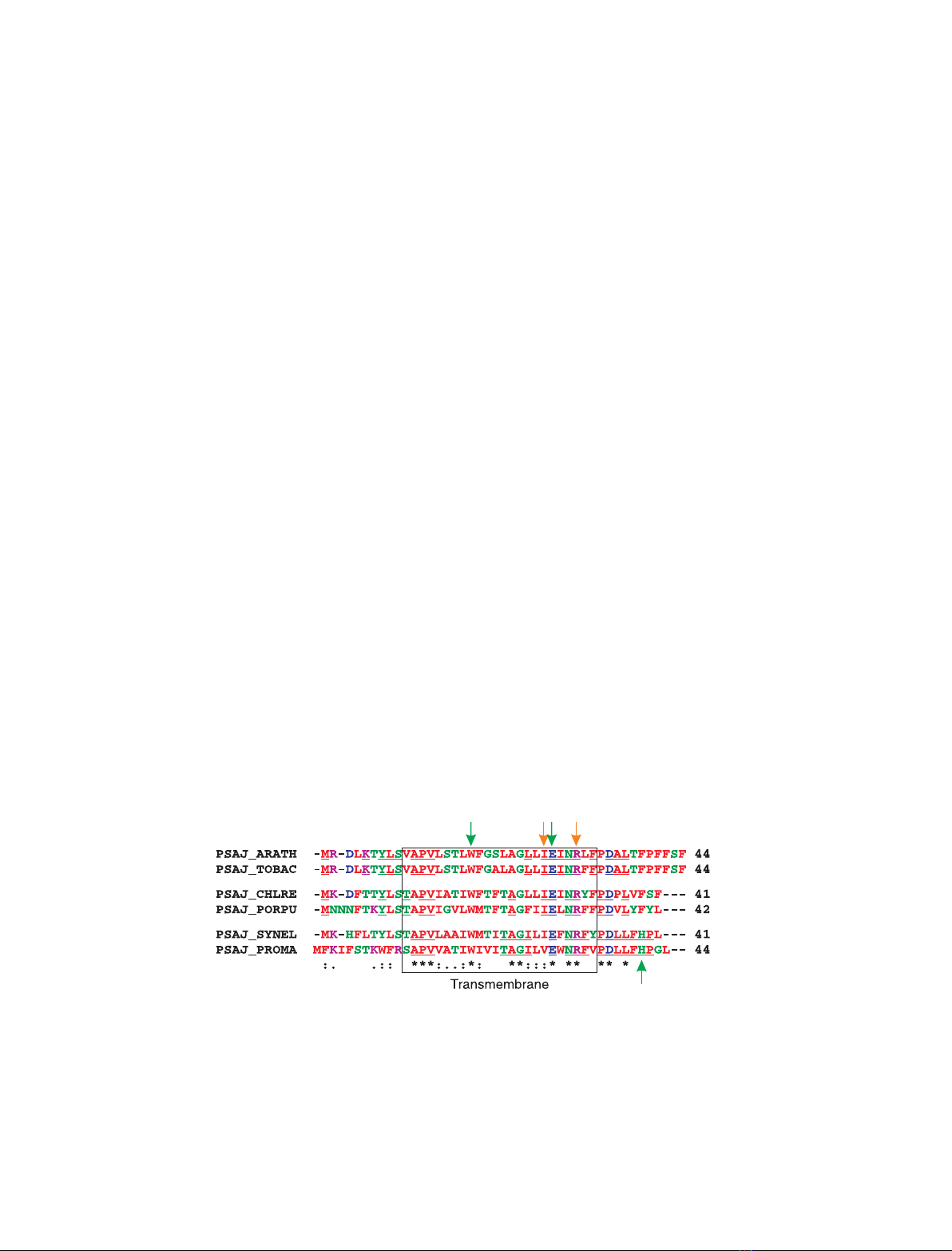
PSI-J is a hydrophobic low-molecular-mass subunit
composed of 44 amino acids with one transmembrane
helix that is located close to PSI-F [5,10]. The N-termi-
nus of PSI-J is located in the stroma, and the C-termi-
nus is located in the lumen [6]. In cyanobacteria, PSI-J
binds three chlorophylls (Chls) and is in hydrophobic
contact with carotenoids [5], whereas in plants only
two Chl molecules are bound (Fig. 1), which has been
proposed to be important for energy transfer between
LHCI and the PSI core [10].
In cyanobacteria, PSI-J interacts with PSI-F [18]. A
psaJ knock-out in Synechocystis PCC 6803 contained
only 20% PSI-F subunit compared with wild-type [19].
The corresponding psaJ knock-out in Chlamydomonas
contained wild-type levels of PSI-F and PSI, and the
cells were able to grow photoautotrophically. A large
fraction of the mutant PSI complexes displayed slow
kinetics of electron donation from Pc or Cyt c
6
to
P700. The absence of PSI-J did not alter the half-lives
of the different kinetic phases, but led to the formation
of two subpopulations of PSI complexes which differed
with respect to electron transfer to P700
+
. One popu-
lation behaved like wild-type with fully functional
PSI-F, and the other population had characteristics
similar to a PSI-F-deficient strain [20]. It was conclu-
ded that, in 70% of the PSI complexes lacking PSI-J,
the N-terminal domain of PSI-F is unable to provide
an efficient binding site for either Pc or Cyt c
6
and was
explained by a displacement of this domain. Thus,
PSI-J does not appear to participate directly in binding
of Pc or Cyt c
6
, but plays a role in maintaining a
precise recognition site for the N-terminal domain of
PSI-F required for fast electron transfer from Pc and
Cyt c
6
to PSI [20].
To determine the role of PSI-J in plants, we gener-
ated homoplastomic psaJ knock-outs in tobacco.
Transplastomic transformants were obtained and ana-
lyzed for differences in electron transport and antenna
function. In contrast with results obtained with PSI-J-
deficient Chlamydomonas, the content of PSI was
reduced by 20% and the remaining PSI had a
decreased in vitro NADP
+
-photoreduction activity. A
secondary loss of the luminal subunit, PSI-N, was seen
when PSI complexes were analysed and kinetic analysis
revealed a large fraction of inactive PSI. Thus, we pro-
pose a dual function of PSI-J in higher plants; one for
assembly of the PSI core complex and the other for
integrity and stabilization of a luminal domain invol-
ving at least PSI-N and the N-terminal part of PSI-F
which is required for efficient electron transfer.
Fig. 1. Alignment of PSI-J sequences representing cyanobacteria, algae and higher plants. In total, 44 full-length PSI-J sequences were
aligned using CLUSTAL W. In the alignment shown are the sequences from plants [Arabidopsis thaliana (ARATH) and Nicotiana tabacum
(TOBAC)], algae [Chlamydomonas reinhardtii (CHLRE) and Porphyra purpurea (PORPU)] and cyanobacteria [Synechcoccus elongatus (SYNEL)
and Prochlorococcus marinus (PROMA)]. Amino-acid residues involved in Chl binding [W (Trp), E (Glu) and H (His)] are indicated with green
arrows. Note that the histidine residue is only conserved in cyanobacteria, in agreement with the notion that PSI-J of cyanobacteria is
involved in binding three Chls, whereas plant PSI-J only binds two. Amino-acid residues making contact with b-carotene [I (Ile) and R (Arg)]
are indicated with orange arrows. The underlined residues are completely conserved in plants, algae and cyanobacteria.
A. Hansson et al.Knock-out of the J subunit of PSI
FEBS Journal 274 (2007) 1734–1746 ª2007 The Authors Journal compilation ª2007 FEBS 1735
Results
Targeted inactivation of the tobacco chloroplast
psaJ gene
To determine the function of PSI-J in plants, we have
taken a reverse genetics approach and constructed a
knock-out allele for targeted disruption of the tobacco
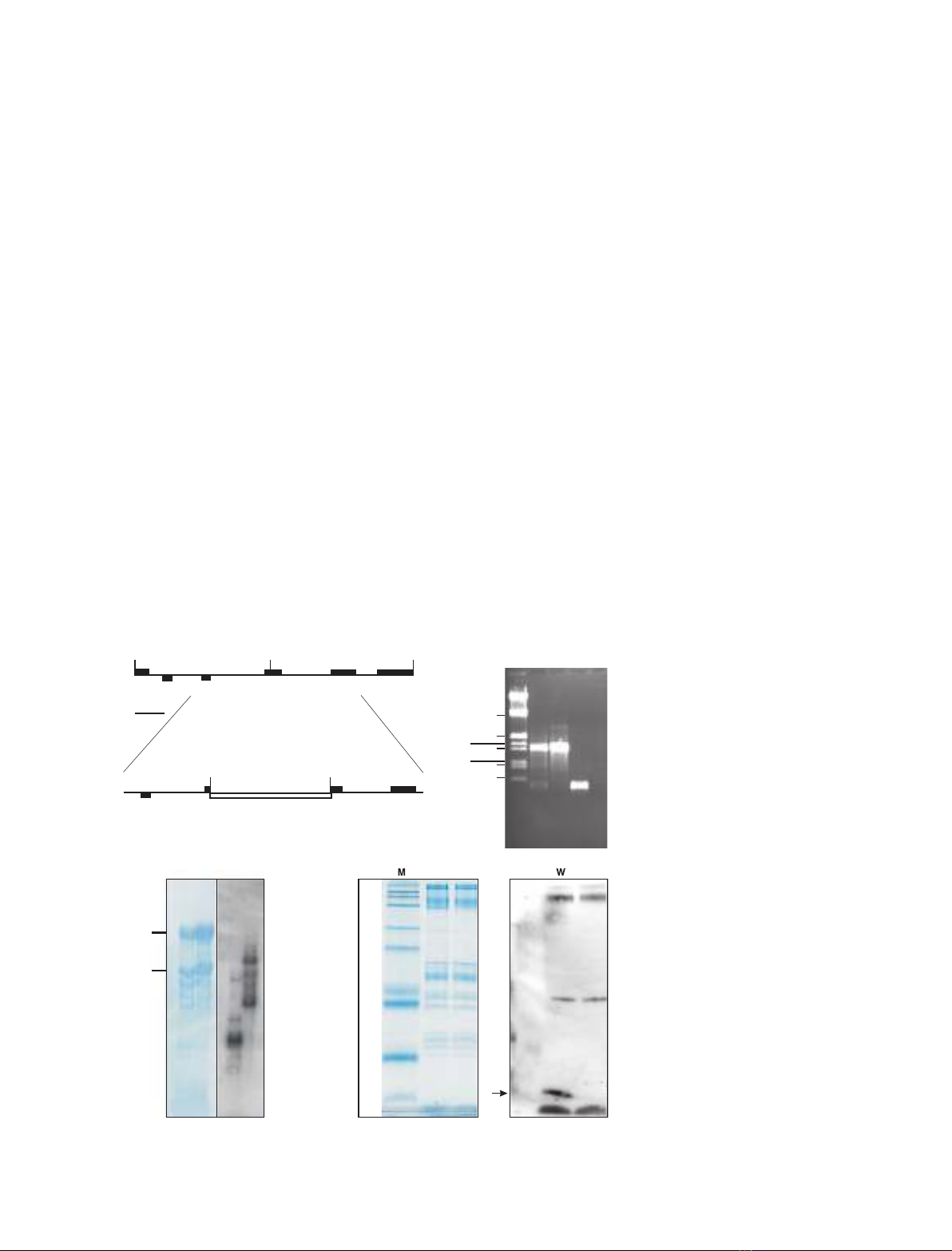
psaJ (Fig. 2A). The knock-out allele was introduced
into the tobacco plastid genome by particle bombard-
ment-mediated chloroplast transformation [21].
From 10 bombarded leaf samples, 19 chloroplast
transformants were selected and verified by PCR
and DNA gel blot analysis (data not shown). Two
independent transplastomic lines were subjected to
additional rounds of regeneration on spectinomycin-
containing medium to obtain homoplastomic tissue. In
Fig. 2B, an example of PCR verification of one of the
homoplastomic psaJ knock-out lines is shown. Nor-
thern blot analysis was also performed to demonstrate
that the psaJ gene was disrupted by the insertion of
the aadA cassette (Fig. 2C). Finally, PSI particles (PSI
holocomplexes) were prepared from wild-type and
plants disrupted in the psaJ gene and subjected to
immunoblot analysis. An antibody originally raised
against electroeluted PSI-I [22] and subsequently found
to recognize both PSI-I and PSI-J [17] was used to
confirm the absence of PSI-J protein from the mutant
(Fig. 2D). Altogether this clearly shows that the psaJ
gene has been disrupted causing elimination of the
PSI-J protein.
Plants devoid of PSI-J are fully viable and fertile
but display a clear phenotype
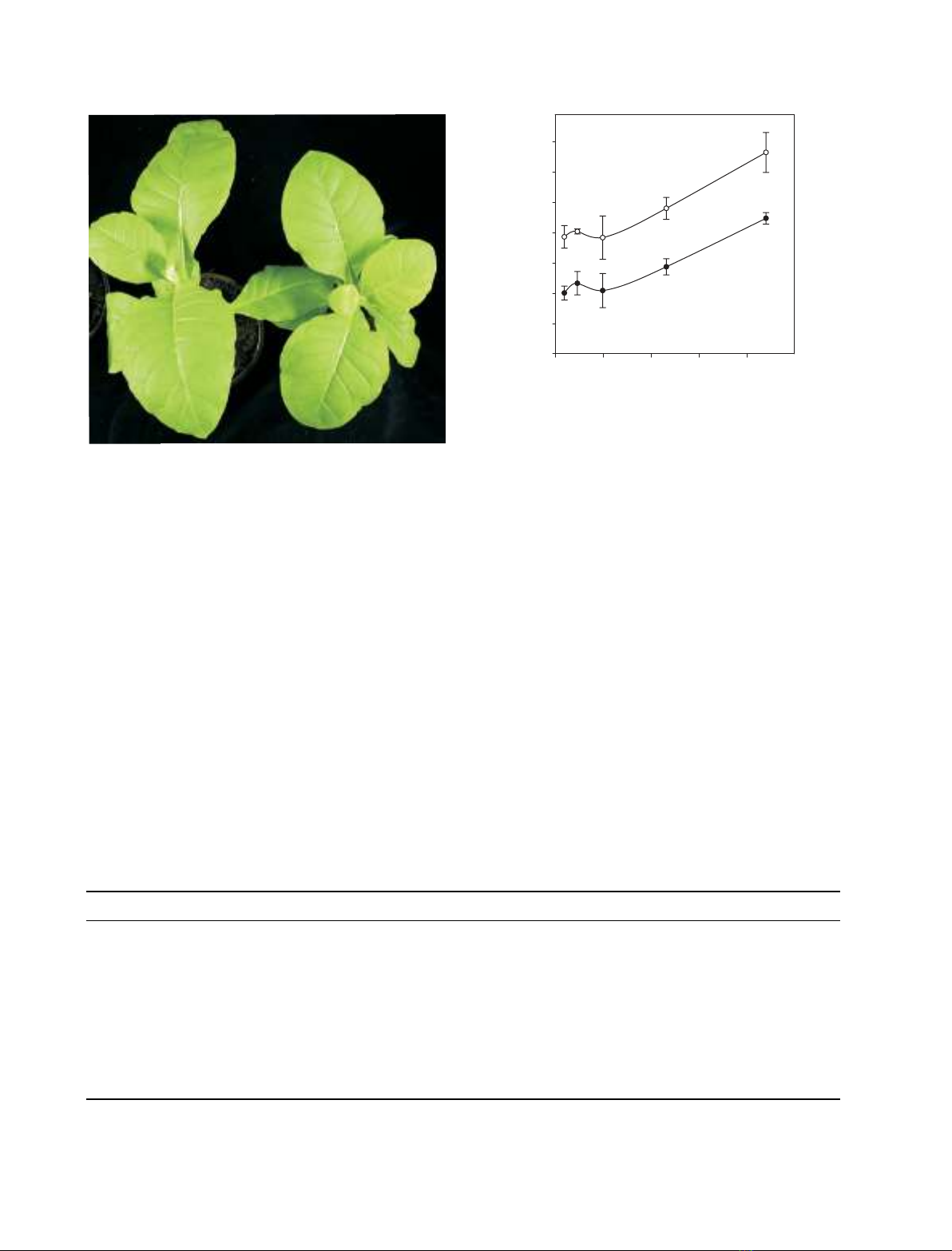
When plants lacking PSI-J were transferred to soil,
they grew photoautotrophically and were fully fertile
(Fig. 3). The original transformed lines were self-polli-
nated, and the seeds produced were germinated
directly on soil. The resulting offspring displayed the
same characteristics as the first generation (results not
shown).
Tobacco plants lacking PSI-J were slightly smaller
than wild-type plants (Fig. 3). This was observed for
plants grown in either a growth-chamber or a green-
house and suggests that elimination of the PSI-J pro-
tein from PSI affects the overall photosynthetic
performance.
Besides being slightly smaller than wild-type, the
psaJ knock-out plants were visibly paler. Pigment
WT ∆JT∆J
M
4
7
16
17
34
45
55
105
kDa
M123
564
947
831
1375
1584
2027/1904
3530
AB
CD
3.7 kb
1.9 kb
WT WT
68293 70823
PetG TrnW TrnP PsaJ Rpl33 Rps18
250-bp
ScaI
TrnP (PsaJ) Rpl33
(ScaI/SmaI)
(PsaJ)
(HindIII/ScaI)
AadA
∆J∆J
Fig. 2. (A) Construction of the plastid trans-
formation vector. Schematic map of the
2.53-kb chloroplast genomic fragment con-
taining the psaJ gene. The aadA cassette is
inserted in a ScaI site within the coding
sequence of psaJ in the sense orientation.
(B) PCR confirmation that the aadA cassette
has inserted in the psaJ gene. M, marker;
1, total DNA from transgenic plant as tem-
plate; 2, plasmid DNA used to transform the
plants as template; 3, total DNA from wild-
type tobacco as template. (C) Northern blot
showing that there is no wild-type-sized
psaJ mRNA (as a loading control the left
hand side shows the stained and the right
hand side the actual Northern blot). (D)
Immunoblot analysis of PSI complexes from
wild-type and DpsaJ plants. The panel on
the left is the stained gel, and the panel on
the right is an immunoblot using an antibody
directed against a mixture of PSI-I and
PSI-J. The arrow indicates PSI-J.
Knock-out of the J subunit of PSI A. Hansson et al.
1736 FEBS Journal 274 (2007) 1734–1746 ª2007 The Authors Journal compilation ª2007 FEBS
extraction of leaf discs using boiling ethanol and spec-
trophotometric quantification showed a 13% reduction
in the content of Chl per leaf area compared with
wild-type (Table 1). Estimated from the leaf extracts,
the Chl a⁄bratio was 2.95 in the psaJ knock-out leaves
compared with 3.25 in the wild-type leaves. This differ-
ence was caused by a bigger decrease in Chl a(15%
less) and a smaller decrease in Chl b(6% less) in the
mutant (Table 1). Similar measurements on several
independent preparations of thylakoids also revealed a
lower Chl a⁄bratio in the mutant, although the abso-
lute numbers were different. The reduced Chl a⁄bratio
suggests that plants without PSI-J either have less of
the core complexes or increased content of the Chl b
containing peripheral antenna.
To monitor the photosynthetic electron flow through
PSI during steady-state photosynthesis in vivo, we esti-
mated the redox state of P700 in the light by measuring
oxidation of P700 in the leaf as DAat 810 minus 860 nm
as described in Experimental procedures. The light
dependence of the P700 oxidation ratio (DA⁄DA
max
)
was examined, and, in both the wild-type and DPSI-J
plants, P700 oxidation was almost linearly related to
increasing light intensity. However, in the DPSI-J plants
the redox state of P700 was higher than wild-type at all
light intensities (Fig. 4). This means that P700 stays
more oxidized in the absence of PSI-J. This usually sug-
gests that electron donation from Pc to P700
+
is affec-
ted. Comparison of the curves suggested that about
20% of the PSI has very inefficient electron donation
from Pc in the absence of PSI-J.
Table 1. Chl aand bcontent per leaf area, Chls per PSI reaction centre, PSI activity, and the plastoquinone redox state under different light
conditions.
Wild-type n DPSI-J n
Chl (lg⁄cm
2
) Leaf 19.1 ± 2.1 6 16.6 ± 1.0* 6
Chl a⁄bLeaf 3.25 ± 0.3 6 2.95 ± 0.1* 6
Chl a(lg) Leaf 14.6 ± 1.8 6 12.4 ± 0.7* 6
Chl b(lg) Leaf 4.5 ± 0.3 6 4.2 ± 0.3 6
Chl ⁄P700 Thylakoids 435 ± 17 3 531 ± 32* 3
NADP
+
photoreduction
a
Thylakoids 24.8 ± 2.0 3 11.1 ± 1.0*** 3
[lmol NADP
+
Æs
)1
Æ(lmol P700)
)1
]
1–q
P
Growth chamber ⁄growth light 0.024 ± 0.003 3 0.04 ± 0.01* 5
1–q
P
Greenhouse ⁄cloudy and rainy 0.013 2 0.019 2
1–q
P
Greenhouse ⁄sunny, no clouds 0.028 2 0.065 2
a
Mean of three independent thylakoid preparations. *P<0.05; ***P<0.001.
WT
∆PsaJ
Fig. 3. Phenotype of homoplastomic DpsaJ plants grown under
growth chamber conditions. Note that the DpsaJ plant is slightly
smaller and paler than the wild-type plant.
Li
g
ht intensity (µE)
0 100 200 300 400
oitar noitadixo
007P
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
WT
∆J
Fig. 4. P700 oxidation state in leaves of wild-type and DpsaJ plants.
Light response of P700 oxidation ratio (DA⁄DA
max
) in leaves of
wild-type (WT) and DPSI-J plants (DJ). All data points are
mean ± SD (n¼3), but in some cases the error bars are covered
by the marker.
A. Hansson et al.Knock-out of the J subunit of PSI
FEBS Journal 274 (2007) 1734–1746 ª2007 The Authors Journal compilation ª2007 FEBS 1737

The PSII excitation pressure (estimated as 1–q
P
) was
subsequently measured in vivo in the growth chamber
under the light conditions to which the plants were
adapted. Under these conditions 1–q
P
was increased
1.7-fold in the plants lacking PSI-J (Table 1), indica-
ting that the PSII excitation pressure was significantly
increased as the result of a more reduced plastoqui-
none pool. Measuring 1–q
P
under greenhouse condi-
tions on either a cloudy or a sunny day confirmed the
higher excitation pressure in plants without PSI-J,
especially under conditions where the plants have to
cope with higher light intensities (Table 1). This is in
agreement with a restriction of electron flow at PSI.
The amount of PSI is reduced in the absence
of PSI-J
To analyze the content of PSI further, the amount of
P700 was determined in solubilized thylakoids using
flash-induced absorption changes in P700 at 834 nm.
The number of Chls per P700 reaction centre was esti-
mated to be 435 ± 17 for wild-type and 531 ± 32 for
thylakoids from the PSI-J-less plants (Table 1). Similar
values were obtained using chemical oxidation and
reduction of P700 (data not shown). This clearly indi-
cates an 20% reduction in P700 in plants lacking
PSI-J.
To investigate this by an independent method and
also to analyze whether the absence of PSI-J caused
changes in photosynthetic complexes, we performed
immunoblot analysis of thylakoid proteins using a
variety of antibodies directed against subunits of the
PSI, PSII and ATP synthase complexes (Fig. 5). The
gels were loaded with proteins corresponding to equal
amounts of Chl. This analysis showed that subunits of
PSII and the ATP synthase were present in amounts
equal or close to the amounts found in wild-type
(Fig. 5). In contrast, the amounts of the analysed sub-
units of the PSI core were consistently reduced by 15–
25% compared with the wild-type (Fig. 5A). This
shows that there are fewer PSI core complexes in the
absence of PSI-J and confirms the spectroscopic deter-
mination of Chl per P700 above. Together this sug-
gests that PSI-J is implicated in stable accumulation of
PSI because of a requirement for this subunit either
during assembly or subsequently for the stability of
the PSI complex.
To analyse the effect of the absent PSI-J in more
detail, immunoblot analysis of PSI particles purified
using sucrose density gradient centrifugation was also
performed (Fig. 6). This revealed that most of the sub-
units analysed were present in the complex of the
mutant in amounts similar to that found in the wild-
type. This included the PSI-F subunit, which is known
to be located next to PSI-J in the complex [5,10]. Sur-
prisingly, the only subunit that was reduced in content
was PSI-N, which was reduced to 30–40% of the wild-
type level.
Fig. 5. Immunoblot analysis of proteins in thylakoids prepared from
DpsaJ and wild-type plants. (A) Content of a range of PSI core pro-
teins and ATP synthase (CF
1
-b). Thylakoids were prepared from
leaves from two to four different wild-type or DpsaJ plants. A dilu-
tion series containing protein corresponding to 1.0, 0.5, and
0.25 lg Chl of the wild-type and 1.0–0.5 lg Chl of the mutant was
separated by SDS ⁄PAGE, blotted and analyzed with the antibodies
indicated. Wild-type (WT) and DpsaJ dilutions were run side by
side, and, for each antibody, the resulting signal was quantified
using the LabWorks software as described in Experimental proce-
dures. Quantification was performed on two independent prepara-
tions of both wild-type and DpsaJ thylakoids. (B) Content of light-
harvesting Chl a⁄bproteins of PSI. Thylakoid proteins were separ-
ated as above and the blots were incubated with antibodies as indi-
cated. The Lhca2 antibody also detects Lhcb4 (CP29). (C) Content
of light-harvesting Chl a⁄bproteins of PSII and PSII core proteins.
Thylakoid proteins were separated as above, and the blots were
incubated with antibodies as indicated.
Knock-out of the J subunit of PSI A. Hansson et al.
1738 FEBS Journal 274 (2007) 1734–1746 ª2007 The Authors Journal compilation ª2007 FEBS


























