Binding of cGMP to the transducin-activated cGMP
phosphodiesterase, PDE6, initiates a large conformational
change involved in its deactivation
Akio Yamazaki
1,2,3
, Fumio Hayashi
4
, Isao Matsuura
5
and Vladimir A. Bondarenko
6
1 Kresge Eye Institute, Wayne State University, Detroit, MI, USA
2 Department of Ophthalmology, Wayne State University, Detroit, MI, USA
3 Department of Pharmacology, Wayne State University, Detroit, MI, USA
4 Department of Biology, Kobe University, Japan
5 Division of Molecular and Genomic Medicine, National Health Research Institutes, Zhunan Town, Taiwan
6 College of Osteopathic Medicine, Touro University, Henderson, NV, USA
Keywords
cGMP binding; cGMP-binding-dependent
protein conformational change; GAF
domains; G-protein-mediated signal
transduction; PDE
Correspondence
V. A. Bondarenko, College of Osteopathic
Medicine, Touro University, Henderson,
NV 89014, USA
Fax: +1 702 777 1799
Tel: +1 702 777 1806
E-mail: vladimir.bondarenko@tun.touro.edu
(Received 30 January 2011, revised 17
March 2011, accepted 22 March 2011)
doi:10.1111/j.1742-4658.2011.08104.x
Retinal photoreceptor phosphodiesterase (PDE6), a key enzyme for photo-
transduction, consists of a catalytic subunit complex (Pab) and two inhibi-
tory subunits (Pcs). Pab has two noncatalytic cGMP-binding sites. Here,
using bovine PDE preparations, we show the role of these cGMP-binding
sites in PDE regulation. Pabcc and its transducin-activated form, Pabc,
contain two and one cGMP, respectively. Only Pabc shows [
3
H]cGMP
binding with a K
d
50 nMand Pcinhibits the [
3
H]cGMP binding. Binding
of cGMP to Pabc is suppressed during its formation, implying that cGMP
binding is not involved in Pabcc activation. Once bound to Pabc,
[
3
H]cGMP is not dissociated even in the presence of a 1000-fold excess of
unlabeled cGMP, binding of cGMP changes the apparent Stokes’ radius of
Pabc, and the amount of [
3
H]cGMP-bound Pabc trapped by a filter is
spontaneously increased during its incubation. These results suggest that
Pabc slowly changes its conformation after cGMP binding, i.e. after for-
mation of Pabc containing two cGMPs. Binding of Pcgreatly shortens the
time to detect the increase in the filter-trapped level of [
3
H]cGMP-bound
Pabc, but alters neither the level nor its Stokes’ radius. These results sug-
gest that Pcaccelerates the conformational change, but does not add
another change. These observations are consistent with the view that Pabc
changes its conformation during its deactivation and that the binding of
cGMP and Pcis crucial for this change. These observations also imply that
Pabcc changes its conformation during its activation and that release of Pc
and cGMP is essential for this change.
Structured digital abstract
lPDE6 alpha,PDE6 beta and PDE6 gamma physically interact by molecular sieving (View
interaction)
Abbreviations
GAF, a domain derived from cGMP-regulated cyclic nucleotide phosphodiesterases, certain adenylyl cyclases, the bacterial transcription
factor FhlA; GTPcS, guanosine 5¢-O-(3-thiotriphosphate); IBMX, 1-methyl-3-isobutylxanthine; OS, outer segments of retinal photoreceptors;
PDE, cGMP phosphodiesterase; PMSF, phenylmethylsulfonyl fluoride; Paand Pb, rod PDE catalytic subunits; Pa¢, cone PDE catalytic
subunit; Pab ⁄Pc,Pab complexes having an unknown number of Pc;Pd, a prenyl-binding protein; Pc, rod PDE inhibitory subunit; Pc¢, cone
PDE inhibitory subunit; T, transducin.
1854 FEBS Journal 278 (2011) 1854–1872 ª2011 The Authors Journal compilation ª2011 FEBS

Introduction
Cyclic GMP phosphodiesterase (EC 3.1.4.17), classified
as PDE6 in the PDE family, is one of the key enzymes
for phototransduction in the outer segments (OS) of
retinal photoreceptors. Its activation is G-protein-med-
iated: illuminated rhodopsin stimulates GTP ⁄GDP
exchange on transducin (T)a, followed by dissociation
of GTP–Tafrom Tbc. The GTP–Taactivates PDE,
resulting in a decrease in the cytoplasmic [cGMP], clo-
sure of cGMP-gated channels and hyperpolarization of
plasma membranes [1–3].
The inactive form of rod PDE is composed of a cat-
alytic subunit complex, Pab, and two inhibitory subun-
its, Pcs, i.e. Pabcc [4–10]. A study using electron
microscopy and image analysis of single particles [11]
shows that bovine Pabcc, 150 ·108 ·60 A
˚, has the
shape of a flattened bell with a handle-like protrusion
(30 A
˚) and that the structure is divided into three
distinct substructures by two holes. Except for the pro-
trusion, the structure also appears to consist of two
homologous structures arranged side by side. These
characteristics are consistent with a model in which
Pabcc’s structure is determined by a dimer of homolo-
gous catalytic subunits consisting of two GAF (a
domain derived from cGMP-regulated cyclic nucleo-
tide phosphodiesterases, certain adenylyl cyclases, the
bacterial transcription factor FhlA) regions and one
catalytic region. Indeed, bovine Pabcc contains two
cGMPs and these bind tightly to substructures formed
by GAF regions [12]. These two substructures, called
the noncatalytic cGMP-binding sites, are similar, but
not identical, in shape and size [11]. This implies that
the manner of cGMP binding to each site and ⁄or the
role of cGMP binding to each site in PDE regulation,
if present, may be different.
The current predominant model for PDE regulation
is simple [13]. For activation, GTP–Tainteracts with
Pcin Pabcc, and the GTP–TaÆPabcc complex, with-
out altering the firm interaction between Pab and Pc,
expresses a high cGMP hydrolytic activity. For deacti-
vation, GTP in the GTP–TaÆPabcc complex is hydro-
lyzed with the help of RGS9 and accessory proteins,
i.e. the GTP is hydrolyzed after formation of a huge
complex, and Pabcc is recovered after dissociation of
various proteins, including GDP-bound Ta(GDP–
Ta). This model conveniently explains the rapid acti-
vation and deactivation of PDE; however, there is no
clear evidence to show a firm and continuous interac-
tion between GTP–Taand Pabcc during Pabcc acti-
vation, as would be shown by the isolation of a
complex of Pabcc with Tacontaining a hydrolysis-
resistant GTP analogue such as guanosine 5¢-O-(3-
thiotriphosphate) (GTPcS). In addition, there is no
definitive evidence to prove the formation of a GTP–
TaÆPabcc complex containing RGS9 and accessory
proteins and its decomposition during deactivation of
GTP–Ta-activated PDE.
Binding of cGMP to the noncatalytic site in Pab is
believed to be involved in PDE regulation. Two mod-
els, the cGMP-regulated Pab-Pcinteraction model
[14–18] and the cGMP-binding direct regulation model
[19], have been proposed to explain the role of cGMP-
binding sites in PDE regulation. In the former model,
the interaction between Pab and Pcis dependent upon
the presence of cGMP at the noncatalytic site. When
the noncatalytic sites of Pabcc are saturated with
cGMP, GTP–Taactivates Pabcc without changing the
tight interaction between Pab and Pc, i.e. a GTP–TaÆ-
Pabcc complex is formed and the complex expresses a
high PDE activity. However, when the noncatalytic
sites are not saturated, GTP–Taactivates Pabcc
through dissociation of Pccomplexed with GTP–Ta,
i.e. a Pc-depleted PDE(s) is produced. Pcin the GTP–
Tacomplex enhances the GTPase activity of Ta; the
resulting GDP–Tainstantly releases Pc, and the
released Pcdeactivates the GTP–Ta-activated PDE. In
the latter model, binding of cGMP to the noncatalytic
sites directly regulates PDE catalytic activity. These
two models appear to explain some observations of
cGMP binding to noncatalytic sites. However, as dis-
cussed later, these models have many ambiguous and
controversial points. Thus, it is difficult to integrate
these concepts smoothly into a coherent model for
PDE regulation.
We have recently challenged the dominant model for
PDE regulation by proposing a new and comprehen-
sive model [11,13,20] in which GTP–Taactivates
Pabcc by forming a complex with a Pc, thereby disso-
ciating the PcÆGTP–Tacomplex. This occurs on mem-
branes and is independent of the cytoplasmic [cGMP].
A significant portion of the PcÆGTP–Tacomplex is
then released into the soluble fraction. Thus, Pabc is
the GTP–Ta-activated PDE. After hydrolysis of GTP,
both soluble and membranous PcÆGDP–Tacomplexes
deactivate Pabc without liberating Pc. These PcÆGDP–
Tacomplexes appear to have a preferential order in
deactivating Pabc. This new model is based on the fol-
lowing observations: (a) Pabc, but not Pab, is isolated
only when OS homogenates are incubated with
GTPcS; (b) the ratio of Pc⁄Pab in Pabcc and Pabc is
2 : 1; (c) the enzymatic activity of Pabc is 12 times
higher than that of Pabcc and is inhibited by 30 nm
Pc; (d) the basic structure of these PDE species is not
A. Yamazaki et al. Roles of cGMP binding in PDE6 regulation
FEBS Journal 278 (2011) 1854–1872 ª2011 The Authors Journal compilation ª2011 FEBS 1855
changed when Pabcc is shifted to Pabc; (e)
PcÆGTPcS–Tais isolated from membranous and solu-
ble fractions; (f) both membranous and soluble
PcÆGDP–Tacomplexes deactivate Pabc without liber-
ating Pc; (g) the membranous PcÆGDP–Tacomplex
appears to be consumed earlier than the soluble
PcÆGDP–Tacomplex; and (h) PDE regulatory mecha-
nisms similar to this model are also found in mamma-
lian and amphibian photoreceptors, as well as in rods
and cones. During these studies, we have also shown
that: (a) the interaction between Pabcc and GTPcS–
Tais short-lived, indicating that GTP–TaÆPabcc is an
intermediate, but not GTP–Ta-activated PDE; (b) free
Pcis not detected in any preparations, implying that
Pcalways forms complexes with other proteins; (c)
Pabccd and Pabcdd are formed when Pabcc and Pabc
are solubilized with Pd, a prenyl-binding protein; (d)
the stoichiometry of Pabccd suggests that only one
lipid moiety may be involved in the interaction of
Pabcc with membranes; and (e) the stoichiometry of
Pabcdd suggests that a lipid moiety in Pab is also
affected by Pcdissociation.
In this study, we extend our model by integrating
the role of cGMP binding to the noncatalytic site. We
demonstrate that Pabcc and Pabc contain two and
one cGMP, respectively, that only Pabc expresses
[
3
H]cGMP-binding activity and that Pcinhibits
[
3
H]cGMP binding to Pabc. We also show that the
cGMP binding to Pabc is suppressed during Pabcc
activation, i.e. cGMP binding is not involved in Pabcc
activation. We also suggest that cGMP binding to
Pabc slowly changes its conformation and that binding
of Pcaccelerates the conformational change. Based on
these studies, we propose that binding of cGMP to
Pabc is the first step in PDE deactivation.
Results
Binding of [
3
H]cGMP to OS membranes
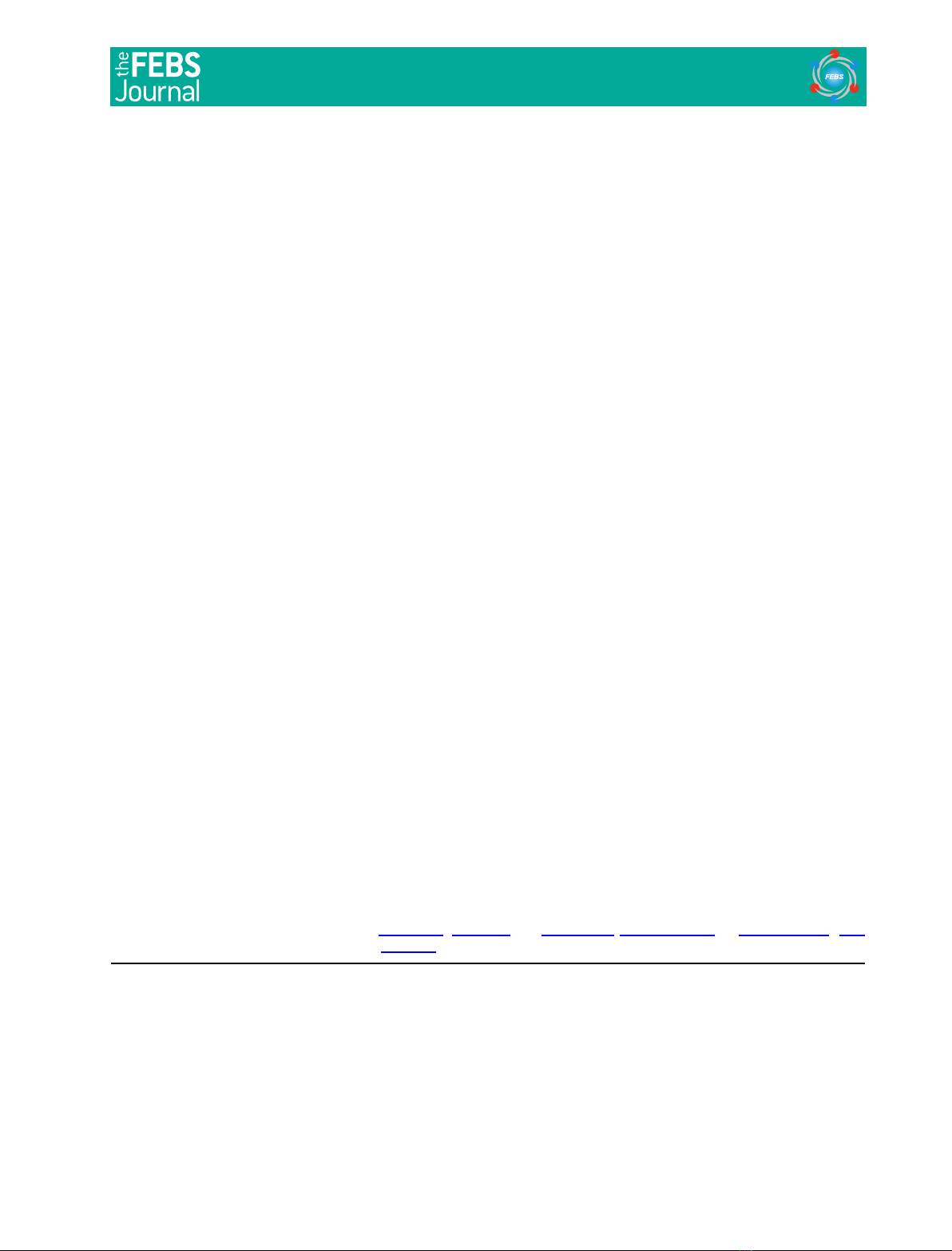
Bovine OS membranes contain a [
3
H]cGMP-binding
site(s) (Fig. 1A). Both GTPcS-treated and nontreated
membranes showed [
3
H]cGMP-binding activities; how-
ever, the activity in GTPcS-treated membranes was
much higher than in GTPcS-nontreated membranes,
indicating that GTPcS–Tasomehow enhances the
[
3
H]cGMP-binding activity. By contrast, the soluble
fraction, whether obtained from GTPcS-treated or
nontreated OS homogenates, showed only negligible
[
3
H]cGMP-binding activity (data not shown). This sug-
gests that no protein in the soluble fraction contains the
[
3
H]cGMP-binding site and ⁄or expresses [
3
H]cGMP-
binding activity under our experimental conditions.
Solubilization and isolation of membranous proteins
showed that a [
3
H]cGMP-binding activity (Fig. 1B)
was detected only in the fraction containing a protein-
doublet (m88 kDa) (Fig. 1C) and that the activity
appeared to be proportional to the level of the pro-
tein-doublet. These fractions also contained a PDE
activity that was proportional to the level of the pro-
tein-doublet (data not shown). The protein-doublet has
been identified as Pab and 70–80% of Pab is extracted
from membranes under these conditions [13,20]. These
results suggest that the [
3
H]cGMP-binding activity in
membranes is due to a Pab complex(s). This implies
that cone PDEs, Pa¢a¢⁄Pc¢complexes, are also present
and that a Pa¢a¢⁄Pc¢complex(s) expresses [
3
H]cGMP-
Fig. 1. Binding of [
3
H]cGMP to membranous PDE. (A) Levels of
[
3
H]cGMP binding to OS membranes treated with or without
GTPcS. OS homogenates (27.5 mg protein) were suspended in
18.4 mL of buffer A and divided into two portions. After incubation
of a portion with 50 lMGTPcS overnight on ice, its membranes
were washed twice with 5 mL buffer A supplemented with 50 lM
GTPcS, twice with 5 mL buffer A and suspended in 5 mL buffer A.
The other portion was treated in the same way but without GTPcS.
Binding of [
3
H]cGMP to these suspensions (10 lL) was assayed
using 1 lM[
3
H]cGMP. (B,C) [
3
H]cGMP binding to proteins extracted
from OS membranes treated with or without GTPcS. OS homogen-
ates (27.7 mg protein) were suspended in 18 mL of buffer A,
divided into two portions and treated with or without GTPcS. Pro-
teins were extracted from membranes with 3 mL buffer B (·7),
concentrated to 0.5 mL and applied to Bio-Gel A 0.5-m column.
[
3
H]cGMP-binding activity (B) and PDE activity (not shown) were
assayed using 60 and 5 lL of the fraction, respectively. Protein pro-
files in the fraction (90 lL) were analyzed by SDS ⁄PAGE and stain-
ing with Coomassie Brilliant Blue (C). The left end lane shows the
molecular mass of standard proteins, 94, 67 and 43 kDa.
Roles of cGMP binding in PDE6 regulation A. Yamazaki et al.
1856 FEBS Journal 278 (2011) 1854–1872 ª2011 The Authors Journal compilation ª2011 FEBS

binding activity. However, neither Pa¢nor its
[
3
H]cGMP-binding activity could be identified. These
failures, we believe, are because of its small abundance
in OS. The soluble fraction also contained a Pab ⁄Pc
complex (peak bin [13]); however, the complex showed
only negligible [
3
H]cGMP-binding activity (data not
shown). This is consistent with the above-mentioned
conclusion that [
3
H]cGMP-binding activity was not
detected in the soluble fraction.
Interestingly, the [
3
H]cGMP-binding activity in
GTPcS-treated PDE was higher than in GTPcS-non-
treated PDE (Fig. 1B). When OS homogenates are
incubated with GTPcS, the Pab content in membranes
is increased 20–30% by binding of the Pab ⁄Pccom-
plex existing in the soluble fraction [13]. Therefore,
binding of the Pab ⁄Pccomplex to membranes and the
resulting expression of a [
3
H]cGMP-binding activity
could increase the activity in membranes. However,
the increase in the activity by GTPcS was much
higher, 2.4 times (Fig. 1B). In addition, Pab in the
Pab ⁄Pccomplex has two cGMP-binding sites at most
[12]. Therefore, we conclude that even if the Pab ⁄Pc
complex could express [
3
H]cGMP-binding activity, the
greater part of the increase is due to an increase in the
activity of a Pab ⁄Pccomplex(s) located on mem-
branes. This is unexpected because previous studies
using frog PDE ⁄membranes [21,22] showed that their
[
3
H]cGMP-binding activity in GTP-nontreated PDE
was much higher than that in GTP-treated PDE. We
also note that this result, with the observation shown
in Fig. 1A, implies that [
3
H]cGMP binding to solubi-
lized PDE species is similar to binding to membranous
PDE species, i.e. the properties of cGMP binding to
membranous PDE species may be estimated by study-
ing cGMP binding to solubilized PDE species.
Identification of PDE species expressing
[
3
H]cGMP-binding activity
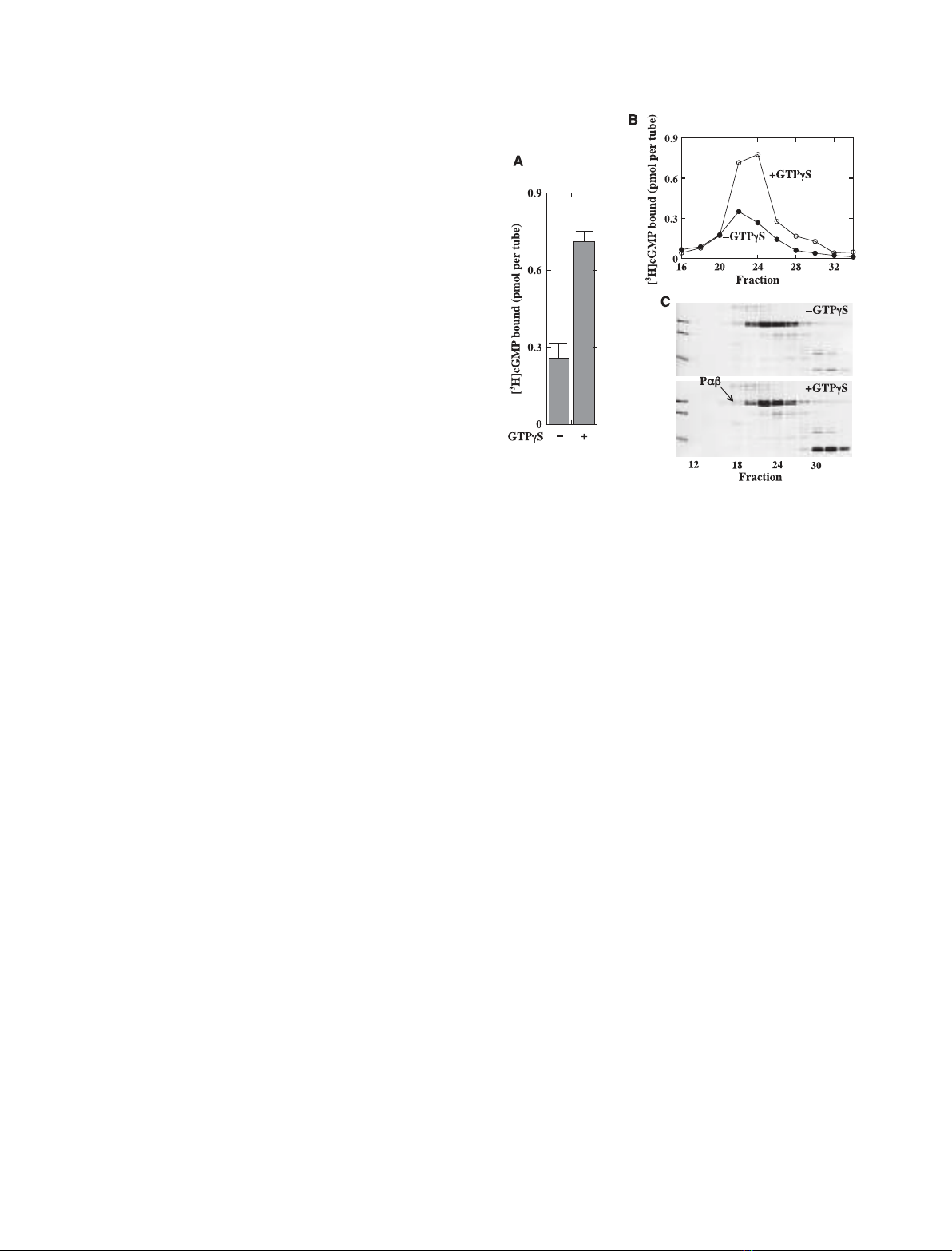
GTPcS-nontreated membranes contain Pabcc, and
GTPcS-treated membranes have Pabcc and Pabc as
major species and a Pab ⁄Pccomplex as a minor species
[20]. These PDE species were extracted using a hypo-
tonic buffer (Fig. 2A) or Pdin an isotonic buffer
(Fig. 2C) and their [
3
H]cGMP-binding activities were
measured after isolation. The use of Pdin an isotonic
buffer may exclude a possible artifact(s) caused by the
hypotonic extraction. OS homogenates were also treated
with GTPcS in the presence of cGMP (GTPcS+
cGMP), and after isolation of Pab ⁄Pccomplexes, their
[
3
H]cGMP-binding activities were measured (Fig. 2B).
The result is compared with the results in Fig. 2A, as
shown later.
Pabcc extracted by a hypotonic buffer
Pabcc was obtained from GTPcS-nontreated mem-
branes (Fig. 2A, upper) and GTPcS-treated membranes
(Fig. 2A, lower). In the former preparation, the
[
3
H]cGMP-binding activity appeared to be proportional
to the level of Pab, implying that Pabcc may express
[
3
H]cGMP-binding activity. However, the molecular
ratio of [
3
H]cGMP to Pab was < 0.01, indicating that
only a negligible portion of the Pabcc expresses this
activity. In the latter preparation, a small [
3
H] radio-
activity was detected in the fraction close to the Pabcc
peak. However, the level of [
3
H] radioactivity was not
proportional to that of Pab in the Pabcc fraction, indi-
cating that the [
3
H] radioactivity is not attributable to
[
3
H]cGMP bound to the Pabcc, i.e. the Pabcc does not
show [
3
H]cGMP-binding activity and ⁄or the Pabcc,
when it exists with GTP–Ta, appears to lose a portion
that may express [
3
H]cGMP-binding activity (Fig. 2A,
upper).
Pabcc extracted with Pdin an isotonic buffer
The Pabccd preparation was obtained from GTPcS-
nontreated membranes (data not shown) and GTPcS-
treated membranes (Fig. 2C). In the former prepara-
tion, the [
3
H]cGMP-binding activity appeared to be
proportional to the level of Pab; however, the molecu-
lar ratio of [
3
H]cGMP to Pab in the Pabccd was
< 0.01. These observations are identical to those for
Pabcc extracted with a hypotonic buffer (Fig. 2A,
upper). In the latter preparation, Pabccd appeared to
show a small [
3
H]cGMP-binding activity (Fig. 2C,
upper). However, the amount of binding was not
exactly proportional to the Pab level in the fraction,
indicating that the [
3
H] radioactivity was not due to
[
3
H]cGMP bound to the Pabccd.
As shown later (Fig. 7), Pabcc can be trapped by a
Millipore filter with a high efficiency, implying that the
lack of [
3
H]cGMP-binding activity and ⁄or the negligi-
ble level of [
3
H]cGMP-binding activity in Pabcc prepa-
rations are not due to the failure to trap [
3
H]cGMP-
bound Pabcc. Taken together, our results strongly
suggest that Pabcc does not express [
3
H]cGMP-bind-
ing activity and that negligible activities occasionally
detected in fractions containing Pabcc may be artifacts
caused by experimental procedures. The level of [
3
H]
radioactivity was not proportional to the level of Ta
(Fig. 2C). This confirms that Tahas no cGMP-binding
site [23]. The amino acid sequence of Taalso supports
this notion. This is specifically noted here because we
use this information in a later discussion.
A. Yamazaki et al. Roles of cGMP binding in PDE6 regulation
FEBS Journal 278 (2011) 1854–1872 ª2011 The Authors Journal compilation ª2011 FEBS 1857
Pabc and Pab ⁄Pc
Whether extracted with the hypotonic buffer (Fig. 2A,
lower) or with Pdin the isotonic buffer (Fig. 2C), frac-
tions containing these PDE species clearly showed
[
3
H]cGMP-binding activities. In addition, the level of
Pab was proportional to that of [
3
H]cGMP-binding
activity in these fractions. These results indicate that
both Pabc and Pab ⁄Pcexpress [
3
H]cGMP-binding
activity.
We emphasize that [
3
H]cGMP-binding activity in the
fraction containing Pabcdd (Fig. 2C, upper) was similar
to that in the fraction containing Pabc (Fig. 2A, lower),
although these activities were apparently different due
to the use of different amounts of OS homogenates and
different volumes of the fraction in the assay. We con-
firmed this observation by comparing the [
3
H]cGMP-
binding activity of Pabc with that of Pabcdd (data not
shown). These results indicate that Pdbinding to the
lipid moiety of Pab does not affect the level of
[
3
H]cGMP-binding activity in Pabc, implying that mem-
brane binding of Pabc may not affect its cGMP-binding
activity. This implication also supports our above-men-
tioned view that properties of cGMP binding to mem-
branous PDE species may be estimated by studying the
cGMP binding to solubilized PDE species. We also note
that the NaCl gradient in the study (shown in Fig. 2C)
was modified to collect both rod and cone PDEs with
fraction numbers similar to those for rod PDEs
(Fig. 2A). Therefore, their elution profile was slightly
different from that shown in Fig. 2A. We have already
shown that the elution profile of PDE species containing
Fig. 2. Binding of [
3
H]cGMP to PDE species extracted from OS membranes. (A,B) PDE species extracted with a hypotonic buffer. Details of
the procedure are given in Experimental procedures. OS homogenates (50.4 mg protein) were suspended in 20 mL buffer A and divided into
three portions. After incubation with cGMP (A, upper), GTPcS (A, lower) or cGMP + GTPcS (B), proteins were extracted with buffer B (a
hypotonic buffer), applied to a TSK–DEAE 5PW column and eluted. Fractions containing PDE species were determined by SDS ⁄PAGE and
assaying PDE activity. Elution profiles of the 88-kDa protein, Pab, are shown in each panel. The elution profile of other proteins is detailed
elsewhere [20]. PDE species were identified as described previously [20]. Binding of [
3
H]cGMP to the fraction (60 lL) was measured with
0.5 lM[
3
H]cGMP. (C) PDE species extracted with Pdin an isotonic buffer. OS homogenates (12.4 mg) were suspended in 13 mL of buf-
fer A and divided into two portions. After incubation of a portion with GTPcS (50 lM) for 1 h on ice, membranes were washed with 2 mL of
buffer A containing GTPcS (50 lM) and 2 mL of buffer A. The other portion was treated in the same way but without GTPcS. These mem-
branes were suspended in 2.5 mL of buffer D, incubated with Pd(final 3 lM) overnight on ice, and washed twice with 2 mL of buffer D. All
supernatants were collected and applied to a TSK–DEAE 5PW column. Rod and cone PDE species and their stoichiometry and transducin
subunits were identified as described previously [20]. Binding of [
3
H]cGMP to the fraction (50 lL) was measured with 0.5 lM[
3
H]cGMP
(upper). Protein profiles in fractions (40 lL) were analyzed by SDS ⁄PAGE and staining with Coomassie Brilliant Blue (lower). Owing to the
limited space, only results from GTPcS-treated membranes are shown. Profiles of PDE species from GTPcS-nontreated membranes are
given in Yamazaki et al. [20].
Roles of cGMP binding in PDE6 regulation A. Yamazaki et al.
1858 FEBS Journal 278 (2011) 1854–1872 ª2011 The Authors Journal compilation ª2011 FEBS


























