
Activity of matrix metalloproteinase-9 against native
collagen types I and III
Heather F. Bigg
1
, Andrew D. Rowan
1
, Michael D. Barker
2
and Tim E. Cawston
1
1 Musculoskeletal Research Group, Institute of Cellular Medicine, The Medical School, Newcastle University, UK
2 Division of Genomic Medicine, Academic Unit of Pathology, University of Sheffield, Medical School, UK
Collagens are the major structural proteins of connect-
ive tissues such as skin, bone, cartilage and tendon.
Interstitial collagen types I, II and III are the most
abundant, and the native triple helical structure of
these molecules makes them highly resistant to proteo-
lysis. However, collagenases of the matrix metallopro-
teinase (MMP) family [1] cleave native collagen
types I, II and III at a specific site in all three chains
of the triple helix, approximately three-quarters of the
length from the N-terminus. The action of these col-
lagenase enzymes is therefore critical for the initiation
of collagenolysis. Once initiated, the cleaved helix
unwinds at physiological temperatures and becomes
susceptible to degradation by other, less-specific pro-
teinases. MMP collagenases are active at neutral pH
and play a highly important role in collagen degrada-
tion in vivo. The mammalian MMP collagenases cur-
rently include the ‘classical’ collagenases, MMP-1,
MMP-8 and MMP-13 [2–4] and also the gelatinolytic
enzyme, MMP-2 [5–7], and MMP-14 (MT1-MMP) [8],
a member of the membrane-type subclass of MMPs.
MMP-9 (also known as gelatinase B, 92 kDa gela-
tinase or 92 kDa type IV collagenase, EC 3.4.24.35)
shares a close structural similarity with MMP-2 [9,10].
It was originally identified as a gelatinolytic enzyme
produced by polymorphonuclear leukocytes [11] and
subsequent studies have demonstrated secretion in the
latent form (proMMP-9) by a variety of cell types. It
has also been implicated in the pathogenesis of several
human diseases, including arthritis [12–15]. Unlike
other MMPs, MMP-9 and MMP-2 both contain three
fibronectin type II repeats inserted into the catalytic
Keywords
arthritis; collagen I; collagen III; collagenase;
matrix metalloproteinase-9
Correspondence
T. E. Cawston, Musculoskeletal Research
Group, 4th Floor, Catherine Cookson
Building, The Medical School, Framlington
Place, Newcastle University, Newcastle-
upon-Tyne, NE2 4HH, UK
Fax: +44 191 2225455
Tel: +44 191 2225397
E-mail: t.e.cawston@ncl.ac.uk
Website: http://www.ncl.ac.uk/medi/
research/rheumatology/
(Received 3 November 2006, revised 20
December 2006, accepted 22 December
2006)
doi:10.1111/j.1742-4658.2007.05669.x
Interstitial collagen types I, II and III are highly resistant to proteolytic
attack, due to their triple helical structure, but can be cleaved by matrix
metalloproteinase (MMP) collagenases at a specific site, approximately
three-quarters of the length from the N-terminus of each chain. MMP-2
and -9 are closely related at the structural level, but MMP-2, and not
MMP-9, has been previously described as a collagenase. This report inves-
tigates the ability of purified recombinant human MMP-9 produced in
insect cells to degrade native collagen types I and III. Purified MMP-9 was
able to cleave the soluble, monomeric forms of native collagen types I and
III at 37 C and 25 C, respectively. Activity against collagens I and III
was abolished by metalloproteinase inhibitors and was not present in the
concentrated crude medium of mock-transfected cells, demonstrating that
it was MMP-9-derived. Mutated, collagenase-resistant type I collagen was
not digested by MMP-9, indicating that the three-quarters ⁄one-quarter
locus was the site of initial attack. Digestion of type III collagen generated
a three-quarter fragment, as shown by comparison with MMP-1-mediated
cleavage. These data demonstrate that MMP-9, like MMP-2, is able to
cleave collagens I and III in their native form and in a manner that is char-
acteristic of the unique collagenolytic activity of MMP collagenases.
Abbreviations
APMA, p-aminophenylmercuric acetate; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinases.
1246 FEBS Journal 274 (2007) 1246–1255 ª2007 The Authors Journal compilation ª2007 FEBS
domain which are thought to mediate the ability to
bind collagen [16,17]. However, this domain does not
appear to be essential for the collagenolytic activity of
MMP-2 [7]. MMP-9, unlike MMP-2, also contains an
additional 54-amino acid proline-rich insertion, homol-
ogous to the a
2
chain of type V collagen [10]. To date,
MMP-9 has not been described as a collagenase. Sev-
eral previous studies have investigated its ability to
digest native collagen types I, II and III using enzyme
from a variety of sources, both natural and recombin-
ant [5,6,18–23]. Three of these demonstrated an inabil-
ity to degrade soluble native collagen I at 22 or 25 C
[5,6,18]; a lack of digestion at 37 C was additionally
reported by Murphy et al. [18]. However, another
study [20] has shown digestion of soluble native colla-
gen I at 30 and 37 C. Four previous studies have
examined the digestion of collagen II and all report no
degradation by MMP-9 [6,18,21,22]. However, investi-
gation into the ability to digest native collagen III has
produced disparate results. Three reports [6,21,22] des-
cribe degradation at 22, 25 or 27 C, whilst two others
[18,20] report digestion at 37 but not 30 C [20] or no
digestion at 25 or 37 C [18]. Furthermore, none of
these previous studies has investigated the initial
MMP-9-mediated cleavage site of collagens I and III.
The possibility of contamination with another MMP
collagenase is very difficult to exclude when working
with a natural source. However production of recom-
binant protein can also present problems with respect
to correct folding of the enzyme, particularly when
prokaryotic cells are used. Both of these issues can be
avoided by expression in insect cells, since these do not
appear to produce collagenolytic metalloproteinases.
This report therefore investigates the ability of purified
recombinant human MMP-9 produced in insect cells
to cleave native collagen types I and III; in addition,
the initial MMP-9-mediated cleavage site of these sub-
strates is investigated for the first time.
Results
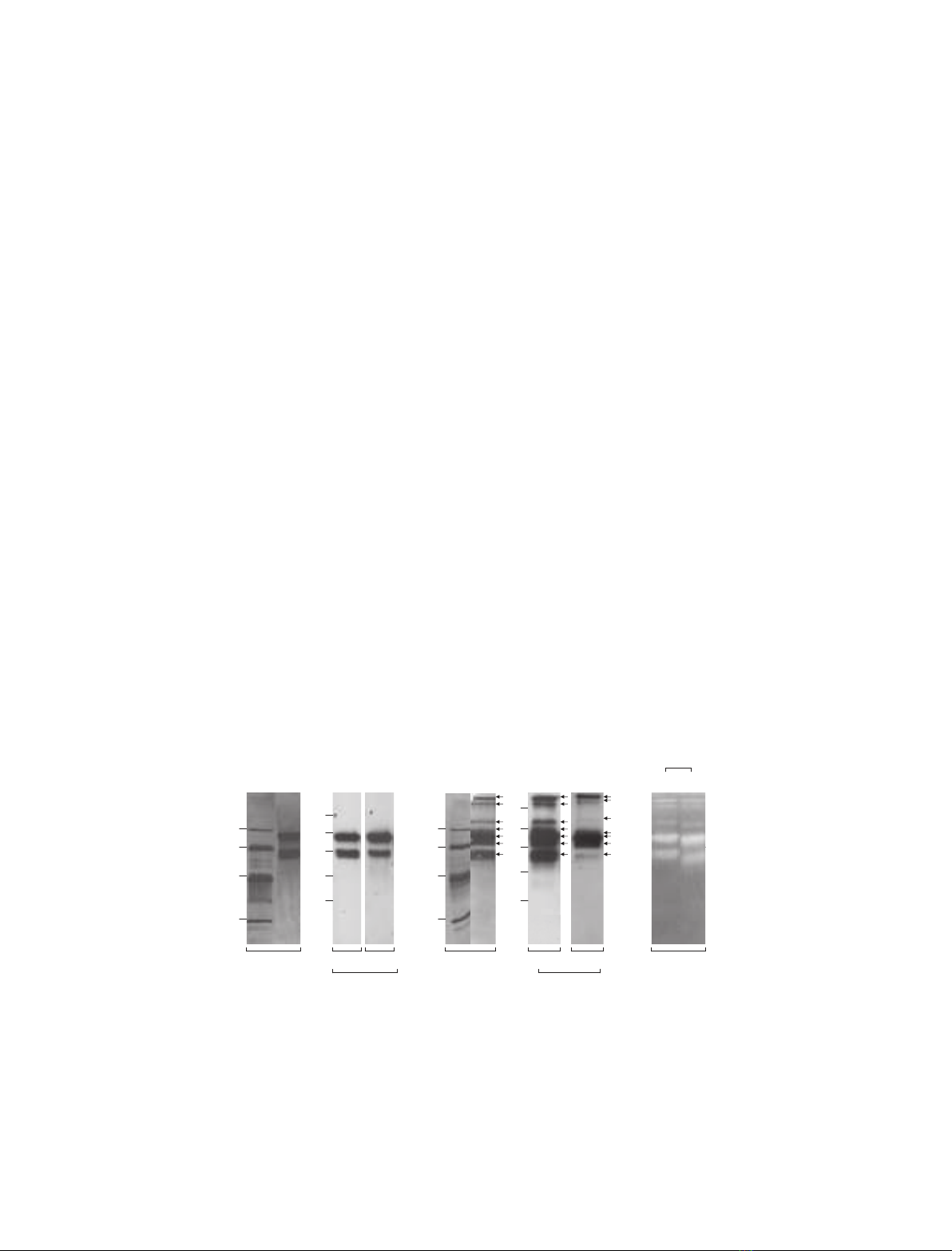
Characterization of purified, recombinant, human
proMMP-9 produced in insect cells
Purified, recombinant, human MMP-9 expressed in
insect cells was used to examine the ability of this
enzyme to cleave native collagens I and III. Recombin-
ant human proMMP-9 was purified from the condi-
tioned medium of pIB-proMMP-9-transfected insect
cells and characterized by silver staining, western blot-
ting and zymography (Fig. 1). Silver staining under
reducing conditions (+bme) revealed two bands
with apparent molecular masses of 85 and 61 kDa,
which were identified as MMP-9 by western blotting
with two anti-MMP-9 sera (Fig. 1A). Human proM-
MP-9 from natural sources has a M
r
of 92 kDa
[10,21,24] and contains both N- and O-linked carbohy-
drate [10]. The molecular mass of the unglycosylated
protein is 81 kDa [10]. The 85-kDa species may
therefore be a differentially glycosylated full-length
form of proMMP-9, but it is also possible that trunca-
tion of the polypeptide occurs during expression and
97.4
66.2
45
MAB911 A560/8
Mr
Mr
(kDa)
(kDa)(kDa)
SILVER
STAIN W. BLOT
+
Mr
97.4
66.2
45
βme
SILVER
STAIN
98
64
50
--
A560/8
(kDa)
Mr
βme
W. BLOT
148
MAB911
-
ZYMOGRAM
APMA
-
--
+
++++
31
148
98
64
50
36
31
36
MrMr
βme
AB C
Fig. 1. Characterization of recombinant human proMMP-9 by silver staining, western blotting and zymography. (A,B) Purified recombinant
human proMMP-9 (0.5 lg) was run reduced (A,+bme) and nonreduced (B,–bme) on 10% SDS ⁄PAGE gels followed by silver staining
(SILVER STAIN) or transfer to nitrocellulose (W. BLOT) as described in Experimental procedures. Western blots were probed with a
polyclonal sheep anti-(porcine MMP-9 serum) (A560 ⁄8, 2 lgÆmL
)1
) and a monoclonal mouse anti-(human MMP-9) serum (MAB911,
2lgÆmL
)1
). M
r
, the positions of molecular mass markers (kDa) are shown. The individual bands present in nonreduced lanes (B) are marked
by arrows. (C) Recombinant human proMMP-9 (1.6 ng) was run nonreduced (– bme) on a 10% gelatine zymogram (ZYMOGRAM) either with
(+) or without (–) prior activation by 0.67 mMAPMA (APMA) for 90 min at 37 C.
H. F. Bigg et al. MMP-9 activity against collagens I and III
FEBS Journal 274 (2007) 1246–1255 ª2007 The Authors Journal compilation ª2007 FEBS 1247

purification. The 61-kDa species is clearly a truncated
MMP-9 fragment, on account of its lower molecular
mass and immunoreactivity to two anti-MMP-9 sera
(Fig. 1A).
Silver staining and western blotting under nonreduc-
ing conditions (– bme) (Fig. 1B) revealed additional
higher molecular mass bands, which therefore appear
to be disulphide-bonded MMP-9 complexes. In addi-
tion, the 85-kDa species displayed heterogeneity when
run nonreduced, since it migrated as three separate
bands; this may result from differential disulphide
bond formation. Zymography (nonreducing condi-
tions, – bme) (Fig. 1C) revealed a similar pattern of
gelatinolytic bands, except that a single band only was
seen for the 85-kDa species. This indicates that the
additional nonreduced forms of this species lack gela-
tinolytic activity, which may be due to incorrect disul-
phide bond formation. The 61-kDa form appears to be
an active site-containing fragment, as it has gelatinoly-
tic activity (Fig. 1C). Activation of the proMMP-9
with p-aminophenylmercuric acetate (APMA) prior to
zymography increased the migration of all species,
including the 61-kDa fragment, therefore demonstra-
ting that all species were proenzyme forms (Fig. 1C).
Purified, recombinant, human MMP-9 cleaves
soluble, native type I collagen
The ability of purified recombinant human MMP-9
produced in insect cells to cleave type I collagen was
tested using soluble substrate at 37 C (Fig. 2). The
collagen retained its native, triple helical structure
under these assay conditions, as it remained resistant
to trypsin (Figs 2A.T). The activity of the trypsin was
confirmed by total lysis of denatured substrate
(Fig. 2A,T,denat). Importantly, preparation of the
type I collagen did not include pepsin digestion, as this
may result in increased susceptibility to gelatinolysis at
37 C. Furthermore, trypsin sensitivity is a reliable
indicator of whether the collagen is susceptible to a
gelatinolytic attack, as progressive heat denaturation
of the collagen at increasing temperatures shows that
resistance to trypsin is lost under the same conditions
as resistance to gelatinolysis (data not shown).
Extensive digestion of the type I collagen band a
chains was seen in the presence of either MMP-1 or
proMMP-9 when combined with APMA. MMP-1-
mediated cleavage did not generate the characteristic
three-quarter length fragments seen at lower incubation
temperatures, because at 37 C these cleavage products
spontaneously denature and are susceptible to further
MMP-1-mediated gelatinolytic degradation. For the
same reason, no fragments at all were detected in the
presence of MMP-9, as this enzyme is a potent
gelatinase. No collagen digestion was seen without
APMA activation of the proMMP-9 (Fig. 2A,
–APMA, + proMMP-9). However, some conversion of
b
12
dimers to a
1
and a
2
monomers is apparent in this
lane, to give an increased level of both the achains and
a slightly increased mobility of the a
2
chain. This indi-
cates the presence of a
2
chain telopeptidase activity,
resulting from a low level of spontaneous proMMP-9
activation during the assay. Digestion of the a
2
N-ter-
minal telopeptides of native type I collagen by MMP-9
has been reported previously [21]. MMP-9-mediated
digestion (both collagenolytic and telopeptidase) was
-+ -+-+ MAB911
proMMP-9
+ APMA MMP-1 MMP-13
β11
β12
α1
α2
buffer
MMP-1
TT
denat
-+
+
+
+
+++
+proMMP-9
APMA
EDTA
serine/
++
+
-
+
++
+
-
--
TIMP-2
EtOH
1,10
mock
β11
β12
α1
α2
cysteine
+
-
mock
A
B
Fig. 2. Recombinant human MMP-9 cleaves soluble, triple helical
type I collagen. (A) Soluble type I collagen from bovine skin (27 lgÆ
lane
)1
) was digested for 72 h at 37 C with buffer alone (buffer),
0.3 lg MMP-1 (MMP-1), 0.2 lg trypsin (T), 0.5 lg recombinant
human proMMP-9 (proMMP-9) or 24 lg of protein from the concen-
trated crude culture medium of mock-transfected insect cells (mock),
in the absence or presence of 0.6 mMAPMA, as indicated. Additional
lanes also contained the following enzyme inhibitors: 6 mMEDTA
(EDTA), 8 mM1,10-phenanthroline (1,10), 2.3 lg TIMP-2 (TIMP-2),
serine and cysteine protease inhibitors at the manufacturer’s recom-
mended working strength (serine ⁄cysteine) or the ethanol solvent
used for 1,10-phenanthroline (EtOH). The efficacy of the trypsin was
demonstrated by cleavage of denatured substrate (denat). The micr-
ogram enzyme–substrate ratio of MMP-9–type I collagen is 1 : 54.
The positions of the uncut collagen band achains (b
11
,b
12
,a
1
and a
2
) are indicated. Cleavage of type I collagen by proMMP-9
combined with APMA was investigated in five separate experi-
ments, with similar results each time. (B) The effect of 4 lg mono-
clonal anti-(human MMP-9) serum (MAB911) on cleavage mediated
by 0.2 lg MMP-1 (MMP-1), 0.2 lg MMP-13 (MMP-13) or 0.1 lg
recombinant human proMMP-9 (proMMP-9) combined with APMA
(+ APMA) is shown. The microgram enzyme–substrate ratio of
MMP-9–type I collagen is 1 : 270. The positions of the uncut colla-
gen band achains (b
11
,b
12
,a
1
and a
2
) are indicated.
MMP-9 activity against collagens I and III H. F. Bigg et al.
1248 FEBS Journal 274 (2007) 1246–1255 ª2007 The Authors Journal compilation ª2007 FEBS

abolished by EDTA, 1,10-phenanthroline and tissue
inhibitor of metalloproteinases (TIMP)-2, but not by
serine and cysteine protease inhibitors, or the ethanol
vehicle for the 1,10-phenanthroline (Fig. 2A), thereby
demonstrating metalloproteinase-mediated activity. No
cleavage was observed in the presence of crude insect
cell culture medium (16.5-fold concentrate) conditioned
by mock-transfected cells (chloramphenicol acetyl
transferase vector) (Fig. 2A, mock), therefore excluding
the possibility of a contaminating insect cell protease.
Furthermore, collagenolytic digestion with a lower level
of MMP-9 was blocked by a monoclonal anti-MMP-9
antibody, whereas cleavage mediated by MMP-1 or
MMP-13 was unaffected or affected only slightly
(Fig. 2B, – ⁄+MAB911). Taken together, these data
convincingly demonstrate that recombinant human
MMP-9 is capable of cleaving native, trypsin-resistant,
soluble type I collagen.
The initial cleavage of type I collagen by MMP-9
is at the three-quarters ⁄one-quarter locus
A hallmark of MMP collagenolytic activity is the abil-
ity to perform the initial cleavage of native substrate
at the three-quarters ⁄one-quarter site. To investigate
the initial cleavage site of MMP-9-mediated type I col-
lagen digestion, we examined its ability to digest
mutated type I collagen which is completely resistant
to collagenolytic cleavage, due to the mutation of
Gln774 (P
2
) and Ala777 (P¢
2
) of the a
1
(I) chain three-
quarters ⁄one-quarter site to proline. The wild-type
a
2
(I) chain of each triple helix is also not cleaved, due
to the presence of two mutated a
1
(I) chains [25].
Mutated type I collagen was not cleaved by MMP-1
or MMP-13, as expected (Fig. 3, mutated, MMP-1,
MMP-13), although telopeptidase activity was evident
in the presence of MMP-1. Under identical conditions,
wild-type collagen I was digested by both these
enzymes; the characteristic three-quarters fragments
are not seen, because at 36 C, these cleavage products
spontaneously denature and are susceptible to further
gelatinolytic degradation. Mutated type I collagen was
also resistant to cleavage mediated by human recom-
binant MMP-9 (Fig. 3, mutated, proMMP-9, APMA).
Under identical conditions, wild-type collagen I was
digested by MMP-9 (Fig. 3, wild-type, proMMP-9,
APMA) to give extensive degradation without the
appearance of partially digested fragments, as for
Fig. 2. The resistance of the mutated type I collagen to
MMP-9-mediated digestion demonstrates that this
enzyme makes the initial cut at the three-quarters ⁄
one-quarter locus, which is a characteristic of MMP
collagenolytic action. Importantly, these data also
exclude gelatinolytic degradation of partially unfolded
wild-type collagen I by MMP-9, as under the same
conditions, this mechanism would also result in suscep-
tibility of the mutated collagen.
MMP-9 cleaves native, triple helical type III
collagen to generate a 3/4 fragment
The ability of recombinant human MMP-9 to cleave
collagen type III was investigated in assays with sol-
uble substrate and compared with the ability to cleave
type I. Type III collagen was cleaved at 25 Cby
recombinant MMP-9, to produce a fragment with a
similar mobility to that of the MMP-1-generated
three-quarter piece (Fig. 4, type III, compare MMP-1
with + proMMP-9, + APMA) [26]. Digestion with
proMMP-9 and APMA was abolished by EDTA,
1,10-phenanthroline and TIMP-2, but not by serine
and cysteine protease inhibitors, or the ethanol vehicle
for the 1, 10-phenanthroline, demonstrating metallo-
proteinase-mediated cleavage. In addition, no digestion
was observed with concentrated crude insect cell
culture medium from mock-transfected cells (Fig. 4,
type III, mock). A low level of cleavage was seen in
the absence of APMA (Fig. 4, type III, – APMA,
+ proMMP-9), indicating some spontaneous activation
of the proMMP-9 during the assay. Minor cleavage
was also seen with trypsin, in agreement with a previ-
ous report demonstrating specific cleavage of native
type III collagen with this enzyme (Fig. 4, type III, T)
[27]. The more extensive digestion of denatured type
III collagen by trypsin (Fig. 4, type III,T, denat)
mutated wild-t
y
pe
buffer
MMP-1
TT
denat
MMP-13
proMMP-9
buffer
MMP-1
TT
denat
MMP-13
proMMP-9
β11
β12
α1
α2
APMA
APMA
β11
β12
α1
α2
Fig. 3. Recombinant human MMP-9 cleaves native type I collagen
at the three-quarters ⁄one-quarter locus. Soluble type I collagen
(27 lgÆlane
)1
) from bovine skin (wild-type) or mouse skin (mutated)
was digested for 98 h at 36 C with buffer alone, 0.6 lg MMP-1,
0.2 lg trypsin (T), 0.5 lg MMP-13 or 0.5 lg recombinant human
proMMP-9 in the additional presence of 0.6 mMAPMA. The effic-
acy of the trypsin was demonstrated by cleavage of denatured sub-
strate (denat). The positions of the uncut collagen band achains
(b
11
,b
12
,a
1
and a
2
) are shown. Cleavage of wild-type and mutated
type I collagen by proMMP-9 combined with APMA was compared
in two separate experiments, with similar results on each occasion.
H. F. Bigg et al. MMP-9 activity against collagens I and III
FEBS Journal 274 (2007) 1246–1255 ª2007 The Authors Journal compilation ª2007 FEBS 1249

confirms that all other conditions represent cleavage
of native rather than denatured substrate. Taken
together, these data clearly demonstrate the ability of
recombinant human MMP-9 to cleave native type III
collagen. Gel-scanning densitometry of the data in
Fig. 4 indicates cleavage of 42% of the type III sub-
strate by MMP-9.
Under the same conditions as the type III assay,
MMP-1 cleaved type I collagen to give characteristic
three-quarter fragments, but no digestion was observed
with proMMP-9 and APMA (Fig. 4, type I).This indi-
cates that recombinant MMP-9 cleaves type III collagen
more effectively than type I, as digestion of type III was
seen at 25 C whereas digestion of type I occurred only
at the higher temperatures of 36 Cor37C (Figs 2 and
3). Recombinant MMP-9 was also able to digest colla-
gen III at 35 and 36 C, as well as at 25 C; however, at
36 C, extensive substrate digestion was also seen in the
presence of trypsin, making it difficult to ascertain that
the collagen retained its native conformation at this tem-
perature (data not shown).
Discussion
A number of previous reports have investigated the
ability of MMP-9 to degrade native collagen types I
and III [5,6,18–23] with disparate results. In this study,
recombinant human MMP-9 was expressed in insect
cells and the ability of enzyme purified from this source
to digest native collagens I and III was evaluated.
Importantly, the possibility of contaminating, endog-
enous collagenolytic activity was excluded, as shown by
the lack of substrate cleavage seen with concentrated,
crude insect cell culture medium from mock-transfected
cells. The data in this report therefore conclusively
demonstrate that MMP-9 is able to digest soluble,
native collagen types I and III at 37 and 25 C, respect-
ively. Furthermore, the location of substrate cleavage
sites was also investigated, demonstrating for the first
time that MMP-9 attacks native collagens I and III
initially at the three-quarters ⁄one-quarter site.
Several previous studies report that MMP-9 is unable
to digest native collagen I [5,6,18,19,23]. In two of these
[19,23], the precise assay conditions are not described
and it is therefore difficult to compare these findings
with the data reported here. Aimes and Quigley [5],
Konttinen et al. [6] and Murphy et al. [18] performed
assays at either 22 or 25 C and reported no digestion
at these temperatures, in agreement with the findings of
this study. The latter study [18] also reported no degra-
dation of native collagen I at 37 C, but these data are
described in the text only and therefore cannot readily
be compared with the data reported here. In agreement
with our study, a further report [20] describes digestion
at both 30 and 37 C, but in this case, the collagen I
substrate was pepsin-treated and therefore possibly sus-
ceptible to a gelatinolytic attack; furthermore, resist-
ance to trypsin was not demonstrated.
Three previous studies [6,21,22] have shown digestion
of native collagen III by MMP-9 at 22, 25 and 27 C,
respectively, in agreement with the data reported here.
However, another study [18] reported no degradation
at either 25 or 37 C. The discrepancy at 25 C may be
due to differences in the quantity of enzyme and assay
period; although the amounts of substrate were similar,
we used more enzyme (4.5·) in a longer assay (5·).
A shorter assay time (5·less) with less enzyme
(0.6·) may also explain the reported lack of digestion
at 30 C [20]; the same study indicated that collagen III
is degraded under these conditions at 37 C.
Of the existing MMPs, MMP-9 is most closely rela-
ted to MMP-2 at the structural level. The C-terminal,
Type III
β11
α1
buffer
MMP-1
TT
denat
APMA-
++++
+
+
EDTA
EtOH
1,10
TIMP-2
proMMP-9
mock
+
++ ++
+
+
serine/
cysteine
mock
-
+-
-
proMMP-9
T
yp
e I
β12
β11
α1
α2
buffer
MMP-1
TT
denat
APMA
Fig. 4. Recombinant human MMP-9 cleaves soluble, triple helical
type III collagen to generate a three-quarters ⁄one-quarter fragment.
Soluble type III or type I collagen (27 lgÆlane
)1
), as indicated, was
digested for 98 h at 25 C with buffer alone, 0.6 lg MMP-1, 0.2 lg
trypsin (T), 0.5 lg recombinant human proMMP-9 or 24 lg of pro-
tein from the concentrated crude culture medium of mock-trans-
fected insect cells (mock) in the absence or presence of 0.6 mM
APMA, as indicated. Additional lanes also contained the following
enzyme inhibitors: 6 mMEDTA, 8 mM1,10-phenanthroline (1,10),
2.3 lg TIMP-2, serine and cysteine protease inhibitors at the manu-
facturer’s recommended working strength or the ethanol solvent
used for 1,10-phenanthroline (EtOH). The efficacy of the trypsin
was demonstrated by cleavage of denatured substrate (denat). The
microgram enzyme–substrate ratio of MMP-9–collagen is 1 : 54.
The positions of the uncut collagen band achains (b
11
,b
12
,a
1
and
a
2
) are indicated. Cleavage of type III collagen by proMMP-9 com-
bined with APMA was investigated in four separate experiments,
with similar results each time.
MMP-9 activity against collagens I and III H. F. Bigg et al.
1250 FEBS Journal 274 (2007) 1246–1255 ª2007 The Authors Journal compilation ª2007 FEBS


























