
Two splicing isoforms of the Y-box protein ctYB-1 appear
on the same mRNA molecule
Dmitry Nashchekin, Sergej Masich, Teresa Soop, Alexander Kukalev, Elizaveta Kovrigina,
Oxana Nashchekina and Bertil Daneholt
Department of Cell and Molecular Biology, Medical Nobel Institute, Karolinska Institutet, Stockholm, Sweden
Y-box binding (YB) proteins constitute a family of
evolutionarily conserved, multifunctional and nucleic
acid-binding proteins [1–3]. They obtained their name
from the early observation that one member of the
family, the human YB-1, bound to the Y-box sequence
in the promoter region of MHC class II genes [4]. Fur-
ther investigations revealed that several YB proteins
are single-stranded, DNA-binding transcriptional fac-
tors involved in the activation and repression of many
genes [5].
Many YB proteins bind to mRNA and affect the
translation of mRNA. These proteins become associ-
ated with mRNA cotranscriptionally and accompany it
from the gene to the cytoplasm [6,7]. It was noted
early on that they are abundant in translationally
repressed (masked) messenger ribonucleoprotein
(mRNP) particles in germ cells of amphibians and ver-
tebrates [8–11]. Later on, it was shown that the YB
proteins are required for male and female fertility in
mammals [12]. YB-1 is a major component of cyto-
plasmic mRNPs in somatic cells as well [13]. Verte-
brate YB-1 is required for optimal translation: it is
essential for initiation of mRNA translation in vitro
[14] and protects mRNA from 5¢-end degradation [15].
However, at high concentrations YB-1 inhibits the
initiation of protein synthesis [16–18]. YB proteins are
Keywords
mRNA transport; mRNP; protein isoforms;
YB-1; Y-box proteins
Correspondence
B. Daneholt, Department of Cell and
Molecular Biology, Medical Nobel Institute,
Karolinska Institutet, S-171 77 Stockholm,
Sweden
Fax: +46 8 313529
Tel: +46 8 524 87370
E-mail: bertil.daneholt@ki.se
(Received 31 August 2006, revised 2
November 2006, accepted 7 November
2006)
doi:10.1111/j.1742-4658.2006.05576.x
Y-box proteins constitute an evolutionarily conserved family of DNA- and
RNA-binding proteins involved in the regulation of transcription and
translation. In the dipteran Chironomus tentans, a homologue to the verte-
brate Y-box protein YB-1 was recently characterized and designated ctYB-1.
It is transferred from nucleus to cytoplasm bound to mRNA and is likely
to affect translation. It appears in two size variants, p40 and p50. We fur-
ther analysed the two size variants and their interaction with mRNA.
Southern blot analysis, in situ hybridization and RT-PCR analysis sugges-
ted that there is just one YB-1 gene, and that the two size variants repre-
sent splicing isoforms. In a C. tentans epithelial cell line, only p40 is
present, whereas both variants appear together in eight tissues from fourth-
instar larvae, although in somewhat different proportions. Furthermore,
the appearance of the two isoforms was studied in relation to a specific 35–
40 kb mRNA transcript in the salivary glands, the Balbiani ring mRNA.
Because of their exceptional size, Balbiani ring messenger ribonucleoprotein
particles in nucleoplasm and Balbiani ring polysomes in cytoplasm could
be identified and selectively studied. We were able to establish that both
isoforms are associated with both nuclear and cytoplasmic Balbiani ring
mRNA. In addition, a p50-specific antibody coimmunoprecipitated p40
from Balbiani ring polysomes, suggesting that the two splicing isoforms are
located along the same Balbiani ring mRNA molecule. The functional sig-
nificance of the two isoforms is being discussed.
Abbreviations
BR, Balbiani ring; CSD, cold shock domain; mRNP, messenger ribonucleoprotein; YB, Y-box binding.
202 FEBS Journal 274 (2007) 202–211 ª2006 The Authors Journal compilation ª2006 FEBS

supposed to exert their effect on translation by modify-
ing the mRNP structure making mRNA more or less
available for translation [10,13].
YB proteins consist of three domains, a middle,
highly conserved, 80 amino acid, cold shock domain
(CSD) surrounded by more variable N- and C-terminal
domains. Remarkably, the vertebrate CSD is > 45%
identical to bacterial cold shock proteins [19]. The
CSD is a five-stranded b-barrel containing RNP1- and
RNP2-like consensus motifs that recognize both DNA
and RNA [2,20,21]. The N-terminal domain is rich in
alanine and proline. In vertebrates and some inverte-
brates, the C-terminal domain contains alternating
clusters of basic and acidic amino acids [6,9]. The
C-terminal tail of YB proteins contributes to the bind-
ing of DNA and RNA and also mediates protein–
protein interactions [2,22].
The N- and C-termini of YB proteins can vary con-
siderably, not only among species, but also within a
given species. More than one gene could be present,
but alternative splicing could also contribute to the
variability. For example, in mouse somatic cells at
least two different YB proteins are expressed, YB-1 ⁄
MSY1 and DBPA ⁄MSY3. Moreover, MSY3 appears
as two splicing variants MSY3S and MSY3L [23]. In
germ cells, the situation is even more complicated.
Here, all three somatic proteins are expressed as well
as two isoforms of the germ-cell-specific MSY2 [11].
Thus, a total of five YB protein variants are expressed
in germ cells and three in somatic cells [23].
The significance of the many YB variants is still
unclear. Many different roles are ascribed to YB pro-
teins and the YB proteins are present throughout
development and in essentially all cell types in the
organism. To better understand the role of each of
individual variant it is essential to study when and
where they appear in the organism and in what context
they are located within the cell.
In this study, we investigated two size variants of
the Y-box protein YB-1 in the dipteran Chirono-
mus tentans, and in particular we analysed the beha-
viour of the variants in salivary glands [24]. The nuclei
of salivary gland cells contain polytene chromosomes
with transcriptionally active regions blown up as puffs.
A few giant puffs, called Balbiani rings (BRs), generate
a transcript of exceptional size (35–40 kb), which is
packed with proteins to an mRNP particle, 50 nm in
diameter. The flow of this large mRNP can be fol-
lowed from gene to polysomes using an electron
microscope [24]. Nuclear BR mRNP particles and
cytoplasmic BR polysomes can be isolated and ana-
lysed for mRNA-associated proteins. The C. tentans
YB-1, designated ctYB-1, has two size variants, p40
and p50 [6]. The variants share the first 258 amino
acids, although the C-termini differ; the p40-specific
terminus comprises six amino acids and the p50-specific
terminus comprises 59 amino acids. It has previously
been shown that ctYB-1 binds mRNA cotransciprio-
nally and accompanies it from the gene to polysomes
[6]. Here, we further study the appearance of the two
variants in the salivary gland cell, in particular in rela-
tion to the BR mRNA. First, we demonstrate that
there is only one YB-1 gene, suggesting that the two
size variants are splicing isoforms. We also show that
the variants are expressed in all larval tissues studied,
although at different proportions. Both variants are
associated with BR mRNA in the nucleus and cyto-
plasm, and they appear together along the same BR
mRNA transcript.
Results
The two ctYB-1 variants are encoded by a single
copy gene
To examine whether the two ctYB-1 size variants are
generated from one or two genes, we performed South-
ern blot analysis and in situ hybridization (Fig. 1). For
Southern blot analysis, C. tentans genomic DNA was
digested with restriction enzymes (Not,EcoRI or Hind
III). Digested DNA was fractionated in an 0.8%
agarose gel and hybridized with a ctYB-1 cDNA probe.
Single bands were observed with Not and HindIII,
which do not cut the ctYB-1 cDNA (Fig. 1A, lanes 1
and 3). Two bands were detected with EcoRI, which
cleaves the ctYB-1 cDNA once (lane 2). In the latter
experiment, most of the cDNA probe corresponded to
the lower DNA band explaining the relatively high
intensity of this band. We conclude that Southern blot
analysis suggested that the ctYB-1 gene is a single copy
gene.
To localize the ctYB-1 gene on C. tentans chromo-
somes, in situ hybridization was performed on polytene
chromosomes using squash preparations of salivary
gland cells. The ctYB-1 cDNA was labelled with dig-
oxigenin and used as a probe and visualized with a
fluorescently labelled digoxigenin antibody. As shown
in Fig. 1B, only a single band was observed, and it
was mapped close to the end of chromosome III
(region 1C)2A). The location of the probe was estab-
lished using cytological analysis of the whole chromo-
some set in 10 specimens (the presence and position of
a nucleolus, distribution of constrictions along the
chromosome and the banding pattern). Taken
together, results from Southern blot analysis and
in situ hybridization show that there is only one ctYB-1
D. Nashchekin et al. Two ctYB-1 isoforms on an mRNA molecule
FEBS Journal 274 (2007) 202–211 ª2006 The Authors Journal compilation ª2006 FEBS 203
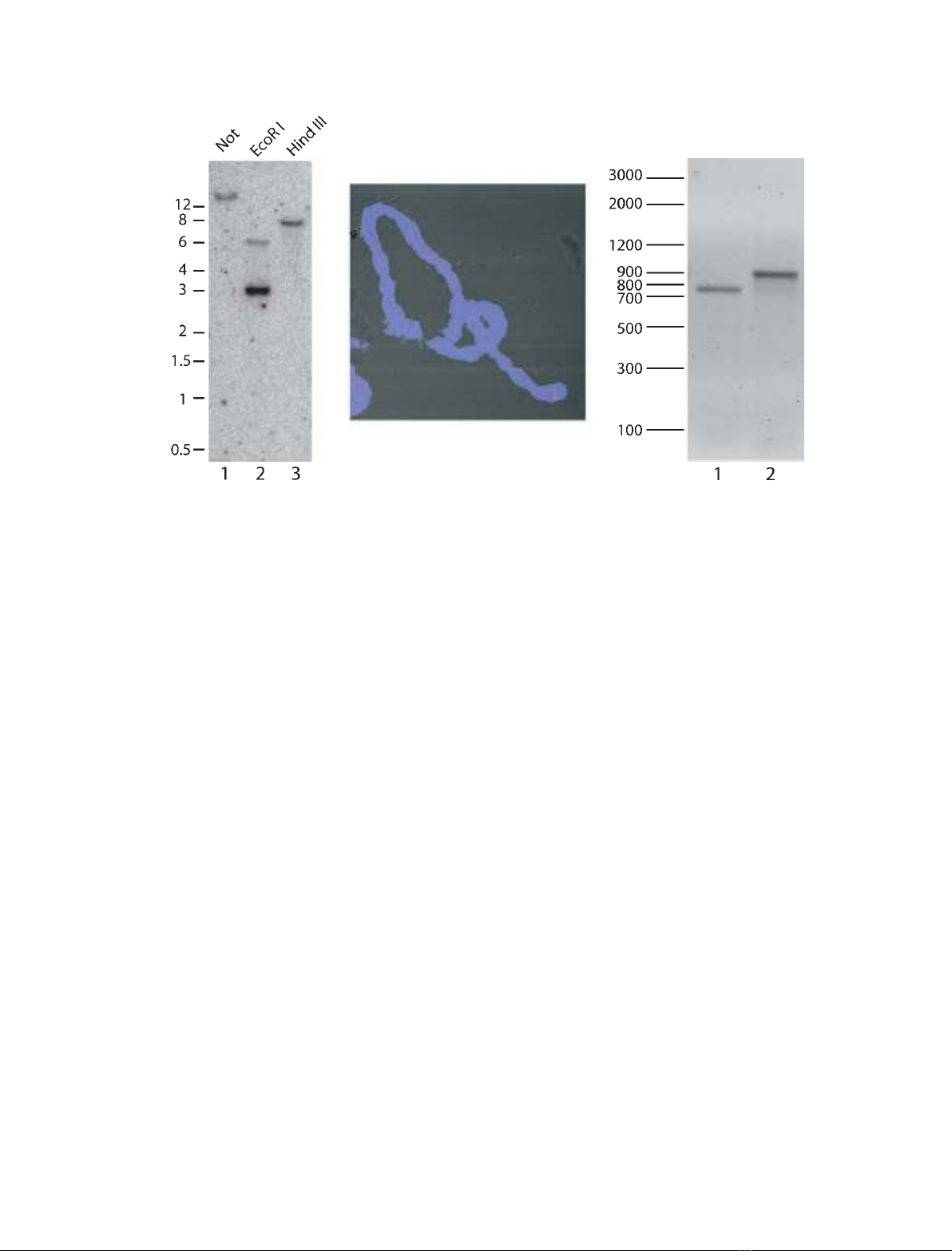
gene, suggesting that the two ctYB-1 variants are enco-
ded in the same gene.
To explore whether there are separate transcripts
corresponding to the two size variants of YB-1, we
performed RT-PCR experiments with salivary gland
RNA and proper primers (see Experimental proce-
dures). As shown in Fig. 1C, we found that the PCR
products corresponded in size to the coding sequence
of p40 mRNA and that of p50 mRNA (lane 1 and 2,
respectively). We conclude that the two YB-1 size vari-
ants are likely to be splicing isoforms.
Both ctYB-1 variants are expressed in many larval
tissues
We have previously shown that p40 alone is present in
tissue culture cells, whereas both p50 and p40 are
expressed in salivary gland cells [6]. To reveal how the
two YB-1 splicing isoforms are expressed in other lar-
val tissues, we prepared protein extracts from various
tissues of C. tentans fourth-instar larvae and carried
out SDS ⁄PAGE and western blot analysis using an
antibody recognizing both the p40 and p50 variants
[6]. As shown in Fig. 2A, p40 and p50 were present in
all samples, but in different proportions (lanes 1–8). In
salivary glands (lane 1), Malpighian tubules (lane 2)
and stomach (lane 3) p50 is the predominant variant,
whereas in intestine (lane 4), colon (lane 5) and ima-
ginal discs (lanes 6–8) both variants are present in
approximately equal amounts. As expected, tissue cul-
ture cells were devoid of the p50 variant (lane 9). The
higher mobility of the two variants in the intestine
sample may be due to divergent processing but is per-
haps more likely caused by site-specific degradation
during preparation of the sample.
When salivary gland extract was treated with alka-
line phosphatase, we noted a slight but distinct shift in
mobility for both p40 and p50, indicating that both
variants are to some extent phosphorylated (Fig. 2B).
ctYB-1 splicing variants are present in nucleus as
well as in cytoplasm and are associated with BR
mRNA in both compartments
It has previously been shown using immunocytology
and immunoelectron microscopy that ctYB-1 is present
in the nucleus and is abundant in the cytoplasm [6].
To also elucidate whether the two splicing variants of
ctYB-1 appear in both compartments, nuclear and
cytoplasmic extracts from salivary gland cells were
studied using SDS ⁄PAGE and western blot analysis
(Fig. 3). Both proteins were present in the nucleus, but
AB C
Fig. 1. Evidence for a single ctYB-1 gene. (A) Southern blot analysis of C. tentans genomic DNA using a
32
P-labelled ctYB-1 cDNA probe.
Genomic DNA was digested with the indicated restriction enzymes and hybridized with the ctYB-1 probe. The positions of the molecular size
markers (in kb) are indicated on the left. (B) In situ hybridization of a digoxigenin-labelled ctYB-1 cDNA probe to C. tentans polytene chromo-
somes. The probe was detected with a rhodamine-labelled anti-DIG Ig. A single red band can be seen close to the end of one chromosome
(III). DNA was stained with DAPI (blue). (C) RT-PCR on salivary gland total RNA using primers specific to p40 (lane 1) and p50 (lane 2) coding
sequences. The positions of the molecular size markers (in bp) are indicated on the left.
Two ctYB-1 isoforms on an mRNA molecule D. Nashchekin et al.
204 FEBS Journal 274 (2007) 202–211 ª2006 The Authors Journal compilation ª2006 FEBS
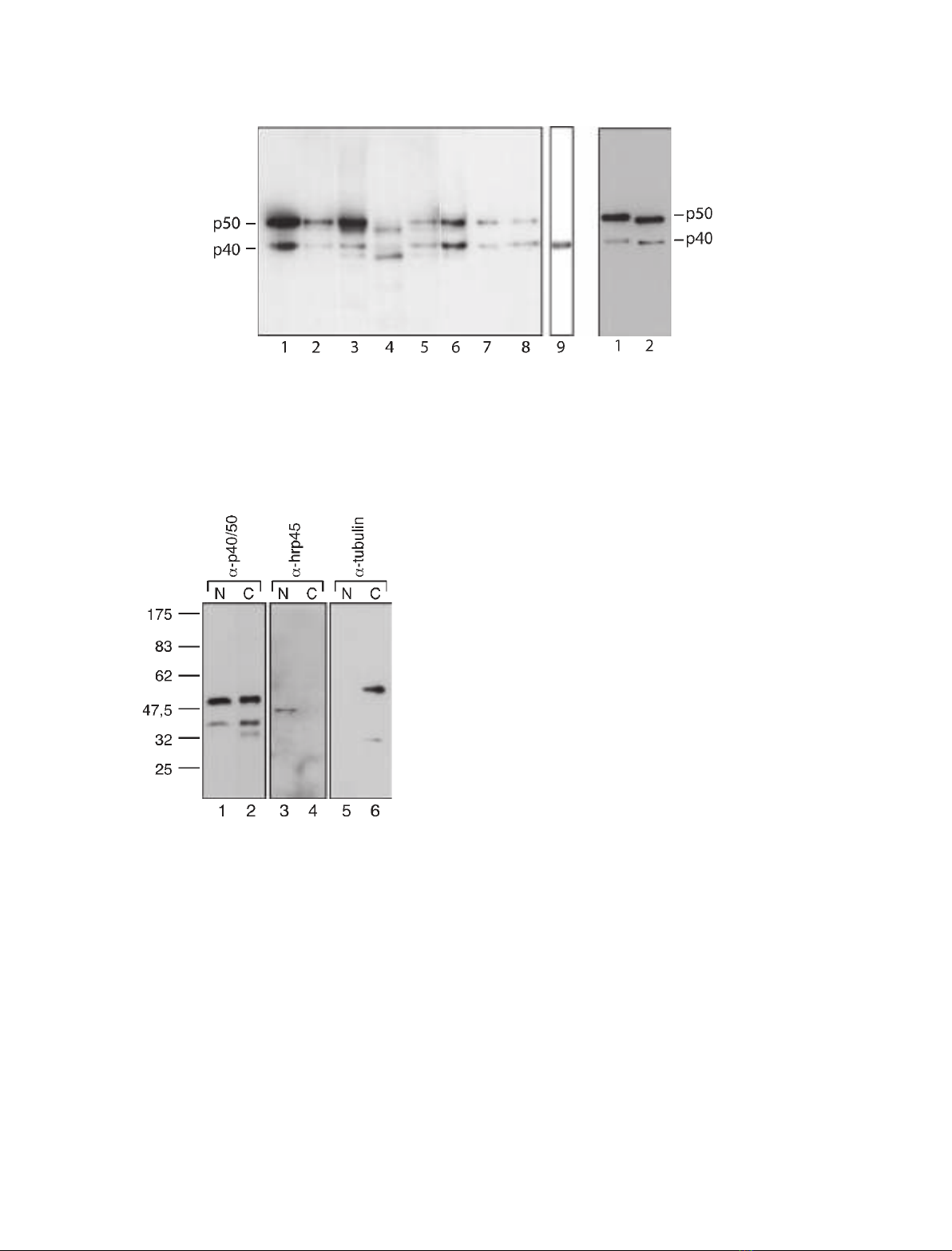
were much more abundant in the cytoplasm (lane 1
versus 2; cf. added amounts). As expected, the control
protein hrp45 was confined to the nucleus (lanes 3–4)
and tubulin to the cytoplasm (lanes 5–6), showing that
there was essentially no cross-contamination between
the nuclear and cytoplasmic samples.
To study the YB-1 variants in relation to a specific
mRNA, nuclear BR mRNP particles were extracted
from salivary gland cells and sedimented in a sucrose
gradient according to Wurtz et al. [27]. The BR RNPs
appeared as a 300Speak (Fig. 4A) and were collected
and bound to oligo(dT) cellulose. The proteins were
released, separated by SDS ⁄PAGE, and analysed by
western blotting using the p40 ⁄50 antibody. Both p40
and p50 could be detected in BR RNP (Fig. 4B). Thus,
both ctYB-1 isoforms copurify with the nuclear BR
RNPs and are likely to be bound to nuclear BR
mRNA.
In the cytoplasm, BR mRNA is associated with
giant BR polysomes (100 ribosomes per polysome)
[28]. Polysomes from salivary gland cells were released
and sedimented in a sucose gradient (Fig. 5A). To ver-
ify the accuracy of the fractionation we examined the
polysomes in one heavy fraction (fraction 8) and one
light fraction (fraction 11) in the electron microscope.
As expected, in fraction 8 we observed giant polysomes
and in fraction 11 smaller ones (Fig. 5A, inserts). Pro-
teins in each tube were examined by SDS ⁄PAGE and
western blot analysis (Fig. 5B). Both p40 and p50 were
present in fractions containing the giant BR polysomes
(Fig. 5B, lanes 7–8) as well as in fractions containing
smaller polysomes (Fig. 5B, lanes 10–11). Both vari-
ants were indeed associated with polysomes, because
disruption of polysomes with EDTA (Fig. 5A) also
shifted p40 and p50 to the top of the gradient
(Fig. 5C). We conclude that the two ctYB-1 splicing
AB
Fig. 2. Tissue distribution of ctYB-1 isoforms. (A) Western blot analysis of ctYB-1 splicing variants in protein extracts from C. tentans tissues.
Extracts were subjected to SDS ⁄PAGE followed by western blotting using a p40 ⁄50 antibody. The following larval tissues were examined:
salivary glands (lane 1), Malpighian tubules (lane 2), cardia chamber of stomach (lane 3), intestine (lane 4), colon (lane 5), imaginal disc from
thorax (lane 6), male genital imaginal disk (lane 7) and female genital imaginal disk (lane 8). An extract from C. tentans tissue culture cells
was studied in parallel (lane 9). Approximately the same amount of protein was loaded in each lane as shown with a Coomassie brilliant
blue-stained control gel (data not shown). (B) Alkaline phosphatase treatment of a protein extract from salivary glands. Lane 1, nontreated
extract; lane 2, treated extract.
Fig. 3. The two ctYB-1 splicing variants are present in both nucleus
and cytoplasm of salivary glands cells from C. tentans. Nuclear (N)
and cytoplasmic (C) samples were extracted from salivary glands
as described in Experimental procedures. The nuclear sample and
1⁄50 of the cytoplasmic sample were subjected to SDS ⁄PAGE and
subsequent western blot analysis. Antibodies against p40 ⁄50
(lanes 1–2), hrp45 (lanes 3–4) and tubulin (lanes 5–6) were used.
The hrp45 protein served as marker for nucleus and tubulin for
cytoplasm. The molecular size (kDa) markers are indicated on the
left.
D. Nashchekin et al. Two ctYB-1 isoforms on an mRNA molecule
FEBS Journal 274 (2007) 202–211 ª2006 The Authors Journal compilation ª2006 FEBS 205
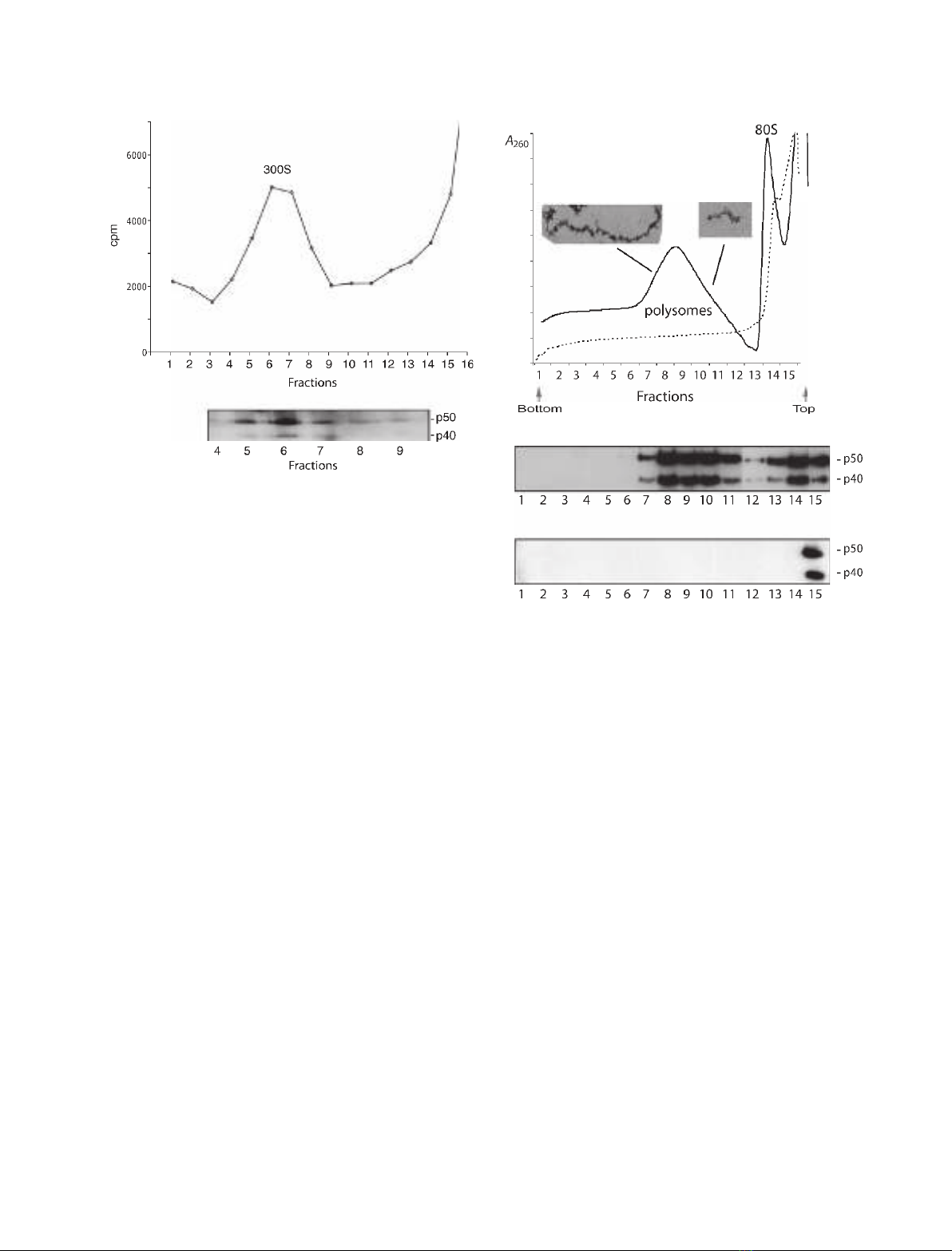
variants in giant polysomes are likely to be associated
with the BR mRNA in polysomes.
The two ctYB-1 splicing variants appear on the
same BR mRNA molecule
To study whether p40 and p50 appear together on the
same BR mRNA molecule, we raised an antibody
against the last 59 amino acids of the C-terminus of
p50. Although the p40 ⁄50 antibody recognized both
proteins in a polysomal extract (Fig. 6A, lane 2), the
p50 antibody stained only p50 (Fig. 6A, lane 1). We
then collected heavy and light polysomes and immuno-
precipitated the polysomes with the p50 antibody
bound to Sepharose beads. The immunoprecipitated
proteins were eluted from the beads, separated by
SDS ⁄PAGE, and analysed by western blotting using
the p40 ⁄50 antibody (Fig. 6B). Both the p40 and p50
variants were precipitated with the p50 antibody from
the heavy (lane 1) as well as light polysomes (lane 2).
The third band in the western blot corresponds to the
heavy chain of the antibody (lane 3). We concluded
that p40 and p50 appear on the same BR mRNA
molecule (heavy polysomes). The ability of the p50
antibody to coimmunoprecipitate p40 not only from
BR polysomes but also from lighter polysomes sug-
gests that the two ctYB-1 variants are also present on
other types of mRNA.
Discussion
In this study we analysed expression of the p40 and
p50 isoforms of the C. tentans Y-box protein ctYB-1.
We have shown using in situ hybridization and South-
ern blot analysis that there is probably only one ctYB-1
gene in C. tentans, suggesting that the two isoforms
A
B
Fig. 4. Both p40 and p50 are recorded in BR mRNP particles. (A)
Sucrose gradient sedimentation of BR RNP particles. Isolated saliv-
ary glands were incubated in the presence of [a-
32
P]ATP, and the
labelled BR RNP particles were released by homogenization and
centrifuged in a sucrose gradient. Fractions were collected from
the bottom of the gradient, and the radioactivity was determined in
each fraction by Cerenkov counting. The BR particles appeared as
a 300S peak in the gradient. (B) Western blot analysis of YB-1 vari-
ants in BR RNPs. The 300Speak fractions (4–9) were incubated
with oligo(dT) cellulose, and the proteins were eluted with sample
buffer, resolved by SDS ⁄PAGE and analysed be western blotting
using a p40 ⁄50 antibody.
A
B
C
Fig. 5. Both p40 and p50 are present in polysomes. (A) Sucrose gra-
dient analysis of polysomes from salivary glands. Polysomes were
extracted from salivary glands and sedimented in a sucrose gradient
as described in Experimental procedures. Fraction numbers are
from bottom to top of the gradient. The absorbance in each fraction
was measured at 254 nm. Electron micrographs of polysomes from
different parts of the gradient are shown as inserts. Untreated
extract (––––); extract treated with 20 mMEDTA (– – –). (B,C) West-
ern blot analysis of YB-1 variants along the sucrose gradient. Pro-
teins in each sucrose gradient fraction were resolved by SDS ⁄PAGE
and analysed by western blot analysis using a p40 ⁄50 antibody.
Untreated extract (B), EDTA-treated extract (C).
Two ctYB-1 isoforms on an mRNA molecule D. Nashchekin et al.
206 FEBS Journal 274 (2007) 202–211 ª2006 The Authors Journal compilation ª2006 FEBS


























