
L-Lactate metabolism in potato tuber mitochondria
Gianluca Paventi, Roberto Pizzuto, Gabriella Chieppa and Salvatore Passarella
Dipartimento di Scienze per la Salute, Universita
`del Molise, Campobasso, Italy
According to the Davies–Roberts hypothesis, plants
primarily respond to oxygen limitation by a burst of
l-lactate production ([1] and refs there in). The acidifica-
tion of the cytoplasm during the first phase of anaerobi-
osis arising from lactic fermentation results in inhibition
of lactate dehydrogenase (LDH) and activation of
pyruvate decarboxylase [2]. As a result, a switch from
lactic to ethanolic fermentation occurs. In those organ-
isms that cannot switch to ethanolic fermentation, when
oxygen falls below 1%, glycolysis is stimulated and
l-lactate accumulates [3], leading to decreased cytoplasmic
pH and cell death [4,5]. Thus, according to the Davies–
Roberts concept, cytoplasmic acidification potentially
induces damage and death of intolerant plants.
Because of the damage that can arise from l-lactate
accumulation, a cellular safety valve to minimize that
damage is to be expected. It has been consistently repor-
ted that metabolism of l-lactate in potato after a period
of anoxia is accompanied by a two-fold increase in
LDH activity and by the induction of two LDH iso-
zymes [6]. These observations related to l-lactate meta-
bolism occurring in the cytoplasm involved pyruvate
formation via LDH, and further pyruvate metabolism,
both in mitochondria and in the cytoplasm. There is rea-
son to suspect, however, that mitochondria themselves
may be involved in l-lactate metabolism. This is based
on our previous work, which has shown that l-lactate is
transported into the organelles isolated from both rat
Keywords
L-lactate; L-lactate dehydrogenase;
mitochondrial transport; plant mitochondria;
shuttle
Correspondence
S. Passarella, Dipartimento di Scienze per la
Salute, Universita
`del Molise, Via De
Sanctis, 86100 Campobasso, Italy
Fax: +39 0 874 404778
Tel: +39 0 874 404868
E-mail: passarel@unimol.it
(Received 2 August 2006, revised 20
December 2006, accepted 10 January 2007)
doi:10.1111/j.1742-4658.2007.05687.x
We investigated the metabolism of l-lactate in mitochondria isolated from
potato tubers grown and saved after harvest in the absence of any chemical
agents. Immunologic analysis by western blot using goat polyclonal anti-
lactate dehydrogenase showed the existence of a mitochondrial lactate
dehydrogenase, the activity of which could be measured photometrically
only in mitochondria solubilized with Triton X-100. The addition of l-lac-
tate to potato tuber mitochondria caused: (a) a minor reduction of intra-
mitochondrial pyridine nucleotides, whose measured rate of change
increased in the presence of the inhibitor of the alternative oxidase salicyl
hydroxamic acid; (b) oxygen consumption not stimulated by ADP, but
inhibited by salicyl hydroxamic acid; and (c) activation of the alternative
oxidase as polarographically monitored in a manner prevented by oxamate,
an l-lactate dehydrogenase inhibitor. Potato tuber mitochondria were
shown to swell in isosmotic solutions of ammonium l-lactate in a stereo-
specific manner, thus showing that l-lactate enters mitochondria by a pro-
ton-compensated process. Externally added l-lactate caused the appearance
of pyruvate outside mitochondria, thus contributing to the oxidation of
extramitochondrial NADH. The rate of pyruvate efflux showed a sigmoidal
dependence on l-lactate concentration and was inhibited by phenylsucci-
nate. Hence, potato tuber mitochondria possess a non-energy-competent
l-lactate ⁄pyruvate shuttle. We maintain, therefore, that mitochondrial
metabolism of l-lactate plays a previously unsuspected role in the response
of potato to hypoxic stress.
Abbreviations
AOX, alternative oxidase; COX IV, subunit IV of cytochrome oxidase; FCCP, carbonyl cyanide p-(trifluoromethoxy)-phenylhydrazone; LDH,
L-lactate dehydrogenase; PTM, potato tuber mitochondria; SHAM, salicyl hydroxamic acid.
FEBS Journal 274 (2007) 1459–1469 ª2007 The Authors Journal compilation ª2007 FEBS 1459
heart [7] and liver [8] and metabolized there. Moreover,
a major role for the mitochondrial LDHs in the transfer
of reducing equivalents from the cytosol to the respirat-
ory chain (lactate shuttle) was also proposed [7].
In order to ascertain whether and how energy meta-
bolism, and in particular l-lactate metabolism, can
change as a result of spontaneous hypoxia in plants,
we used potato, which is an important crop whose
tubers show a high sensitivity to O
2
deprivation [3].
We show here for the first time the existence of LDH
in isolated potato tuber mitochondria (PTM). This
enzyme is localized in the inner mitochondrial
compartments and uses NADP
+
as a cofactor, the
product, NADPH, being oxidized essentially by the
alternative oxidase (AOX), which is activated by pyru-
vate. The latter can also exit from the mitochondria in
a novel l-lactate ⁄pyruvate shuttle operating in a non-
energy-competent manner.
Results
The existence of LDH in mitochondria isolated
from potato tubers
In order to verify the occurrence of LDH in PTM, use
was made of goat polyclonal antibodies raised against
LDH, which have already been shown to cross-react
with LDHs from different species [9–11]. Solubilized
mitochondrial proteins were analyzed by SDS ⁄PAGE,
blotted onto poly(vinylidene difluoride) membrane, and
then probed with the antibody to LDH. In agreement
with Hondred & Hanson [12], LDH protein was visual-
ized as a single band with a molecular mass of about
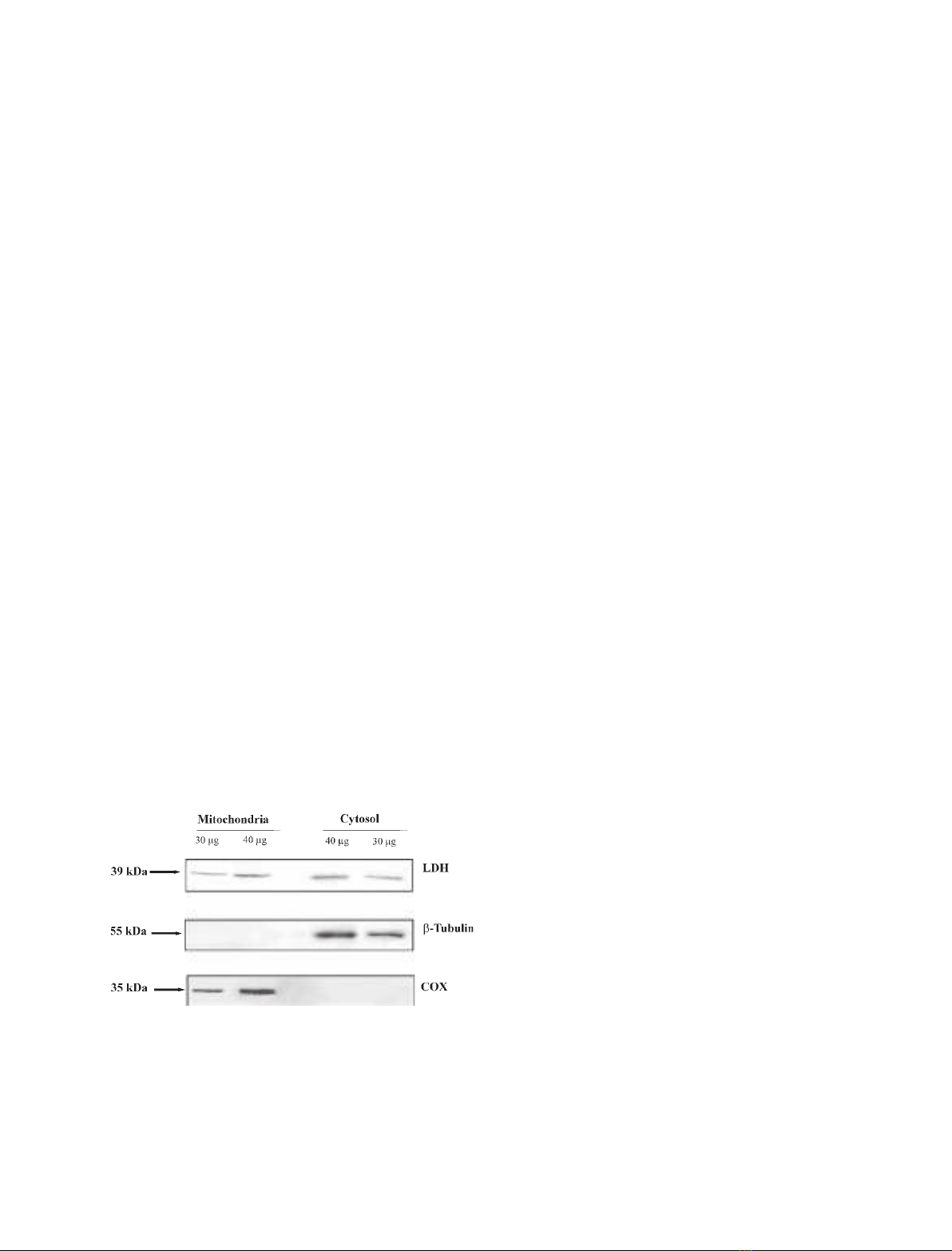
39 kDa. A typical experiment is reported in Fig. 1,
which shows clearly the presence of LDH in the
mitochondrial fraction. Confirmation of this site of ori-
gin was provided by use of a specific antibody against
subunit IV of the cytochrome coxidase (COX IV). A
band corresponding to a protein of molecular mass
35 kDa was observed; this is likely to arise from an
aggregate of COX IV (13 kDa [13]) and other unidenti-
fied protein ⁄s, as already shown in pea mitochondria
[14]. The occurrence of respirasomes in potato mito-
chondria has been recently reported [15], making poss-
ible the occurrence of aggregates not separated in the
SDS ⁄PAGE procedure. Whatever its origins, the lack
of this band in the cytosolic fraction showed that the
35 kDa band is specific for PTM and not a technical
artefact. In the same experiment, it was shown that the
PTM fraction did not contain b-tubulin, a protein
restricted to the cytoplasm, thus ruling out the possibil-
ity that the LDH detected arose from cytosolic contam-
ination. Contamination by other particulate ⁄membrane
fractions was also ruled out, as we used purified mito-
chondria free of subcellular contamination (see Experi-
mental procedures).
The cytosolic fraction was free of mitochondrial
COX IV, showing that minimal rupture of PTM had
occurred during isolation. The intactness of the mit-
ochondrial outer membrane was measured as in Douce
et al. [16], and found to be 95%. In addition, we found
negligible fumarase activity, a plant mitochondrial
marker [17], in suspensions of mitochondria, thus fur-
ther confirming the intactness of the inner membrane.
To establish where LDH is localized within the
mitochondria and whether it is active, LDH was
assayed photometrically by measuring the absorbance
decrease of NADH [18] in the presence of pyruvate in
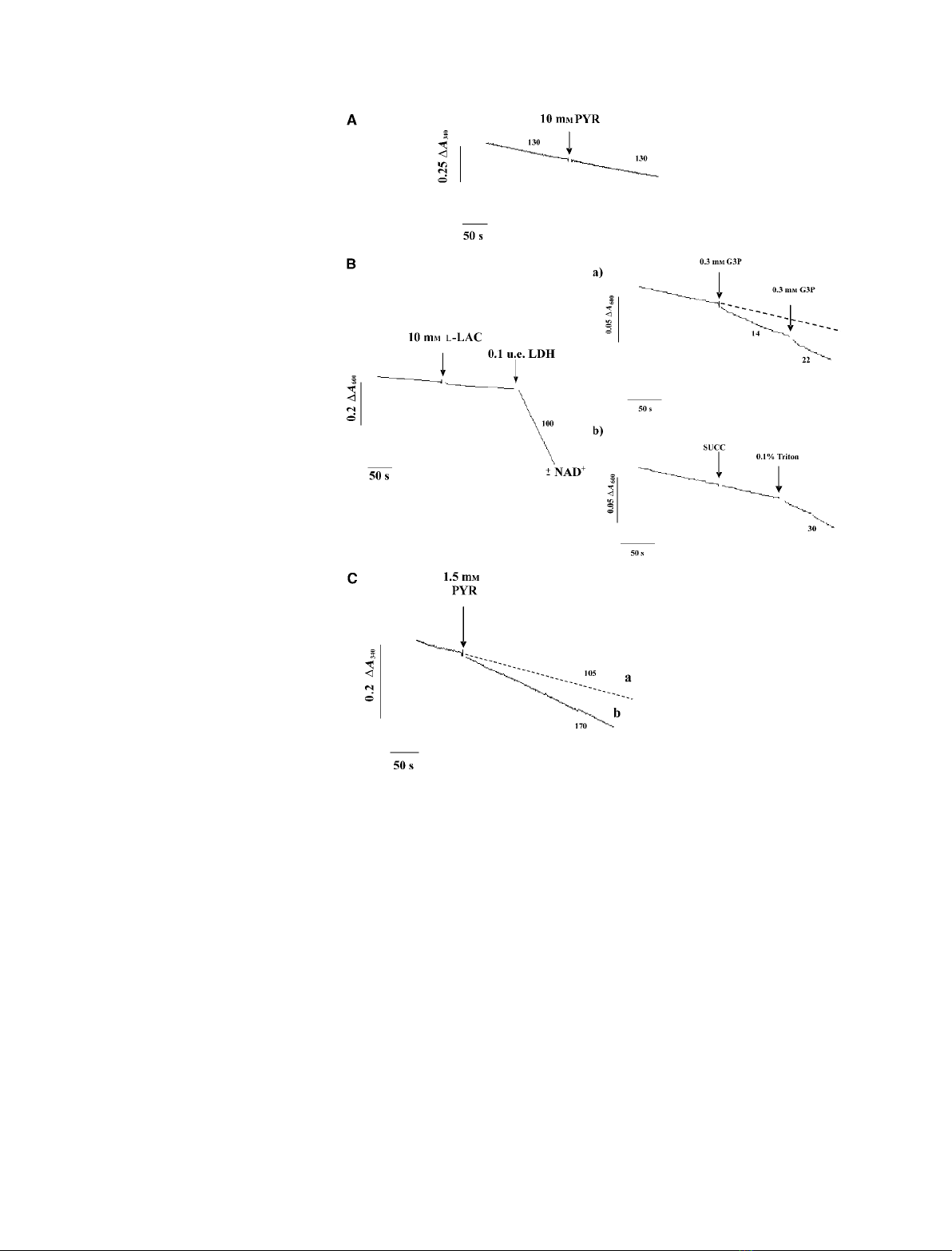
isolated PTM. When PTM (0.1 mg protein) were incu-
bated in the presence of NADH (0.2 mm), oxidation
occurred, catalyzed by external NADPH dehydro-
genases (Fig. 2A). The constant rate of decrease
in absorbance (about 130 nmolÆmin
)1
Æmg protein)
remained unchanged when pyruvate (10 mm) was
added; that is, the LDH was not accessible to sub-
strates. Consistently, no NADH formation was found
in the presence of 10 mml-lactate (not shown).
In order to rule out the possibility that l-lactate is
oxidized on the external face of the inner membrane,
with electrons transferred to the inner surface, intact
PTM were assayed for LDH activity by using phena-
zine methosulfate and dichloroindophenol (Fig. 2B), as
in Atlante et al. [19]. A negligible decrease in dichloro-
indophenol absorbance at 600 nm was found when
l-lactate (10 mm) was added to the PTM, either in the
absence or in the presence of 1 mmNAD
+
, confirming
the absence of LDH activity in the outer membrane, in
Fig. 1. Immunodetection of mitochondrial LDH. Solubilized protein
(30 and 40 lg) from both mitochondrial and cytosolic fractions was
analyzed by western blot as described in Experimental procedures.
Membrane blots were incubated with polyclonal anti-LDH, anti-
COX IV and anti-b-tubulin. COX IV and b-tubulin were used as mit-
ochondrial and cytosolic markers, respectively.
L-Lactate metabolism in PTM G. Paventi et al.
1460 FEBS Journal 274 (2007) 1459–1469 ª2007 The Authors Journal compilation ª2007 FEBS
the intermembrane space or on the outer side of the
mitochondrial inner membrane, or in any contamin-
ation of the mitochondrial suspension. Addition of
LDH externally produced a rapid decrease in absorp-
tion by dichloroindophenol. To validate the experi-
mental protocol that we had used, we confirmed that
addition of 0.3 mmglycerol 3-phosphate to PTM in
the presence of phenazine methosulfate and dichloroin-
dophenol resulted in a decrease of dichloroindophenol
absorbance with a rate of about 22 nmolÆmin
)1
Æmg
)1
protein, arising from the activity of glycerol 3-phos-
phate dehydrogenase (EC 1.1.1.8), which is located on
the outer side of the mitochondrial inner membrane
(Fig. 2B,a). On the other hand, no oxidation of succi-
nate by succinate dehydrogenase (which is located on
the matrix side of the inner mitochondrial membrane)
occurred with intact PTM. Oxidation did occur after
the addition of 0.1% Triton X-100, which solubilized
the mitochondrial membranes and allowed the interac-
tion between dichloroindophenol and the succinate
dehydrogenase complex (Fig. 2B,b).
To confirm that LDH is located in the internal mit-
ochondrial compartments, i.e. in the inner face of the
mitochondrial membrane or in the matrix, PTM were
solubilized with Triton X-100 (0.2%). Added NADH
(0.2 mm) was oxidized at a rate of about 105 nmolÆ
min
)1
Æmg
)1
protein, but when pyruvate was added, this
rate increased to about 170 nmolÆmin
)1
Æmg
)1
protein
(Fig. 2C), showing that LDH is present in the inner
mitochondrial compartments.
The kinetic characteristics of the LDH reaction were
studied by determining the dependence of the rate of
oxidation of NADH on increasing concentrations of
externally added pyruvate in solubilized mitochondria
Fig. 2. Mitochondrial LDH activity assay in
PTM. (A) PTM (0.1 mg) were incubated in
2 mL of the standard medium (see Experi-
mental procedures) containing 200 lM
NADH, and the absorbance (A
340
) was con-
tinuously monitored. Pyruvate (PYR, 10 mM)
was added at the time indicated by the
arrow. The numbers alongside the traces
refer to the rate of oxidation of NADH in
nmolÆmin
)1
Æmg
)1
protein. (B) PTM (0.2 mg)
were incubated in 2 mL of standard medium
in the presence of phenazine methosulfate
(PMS) (30 lM) plus dichloroindophenol
(50 lM), either in the presence or in the
absence of NAD
+
, and the absorbance
(A
600
) was continuously monitored. At the
times indicated by the arrows, L-lactate
(L-LAC, 10 mM) and LDH (0.1 eu) were
added. The insets show control experi-
ments: at the times indicated by the arrows,
glycerol 3-phosphate (G3P, 0.3 mM) (a) and
succinate (SUCC, 5 mM) and Triton X-100
(0.2%) (b) were added to mitochondria trea-
ted with phenazine methosulfate and dichlo-
roindophenol. Numbers along the curves are
rates of L-lactate, succinate or glycerol
3-phosphate oxidation expressed as nmol
dichloroindophenol reducedÆmin
)1
Æmg
)1
pro-
tein. (C) PTM solubilized with Triton X-100
(0.2%) were incubated in 2 mL of the stand-
ard medium, containing 200 lMNADH, and
the absorbance (A
340
) was continuously
monitored. Pyruvate (1.5 mM) was added at
the time indicated by the arrow. The num-
bers alongside the traces refer to the rate of
oxidation of NADH in nmolÆmin
)1
Æmg
)1
protein.
G. Paventi et al.L-Lactate metabolism in PTM
FEBS Journal 274 (2007) 1459–1469 ª2007 The Authors Journal compilation ª2007 FEBS 1461
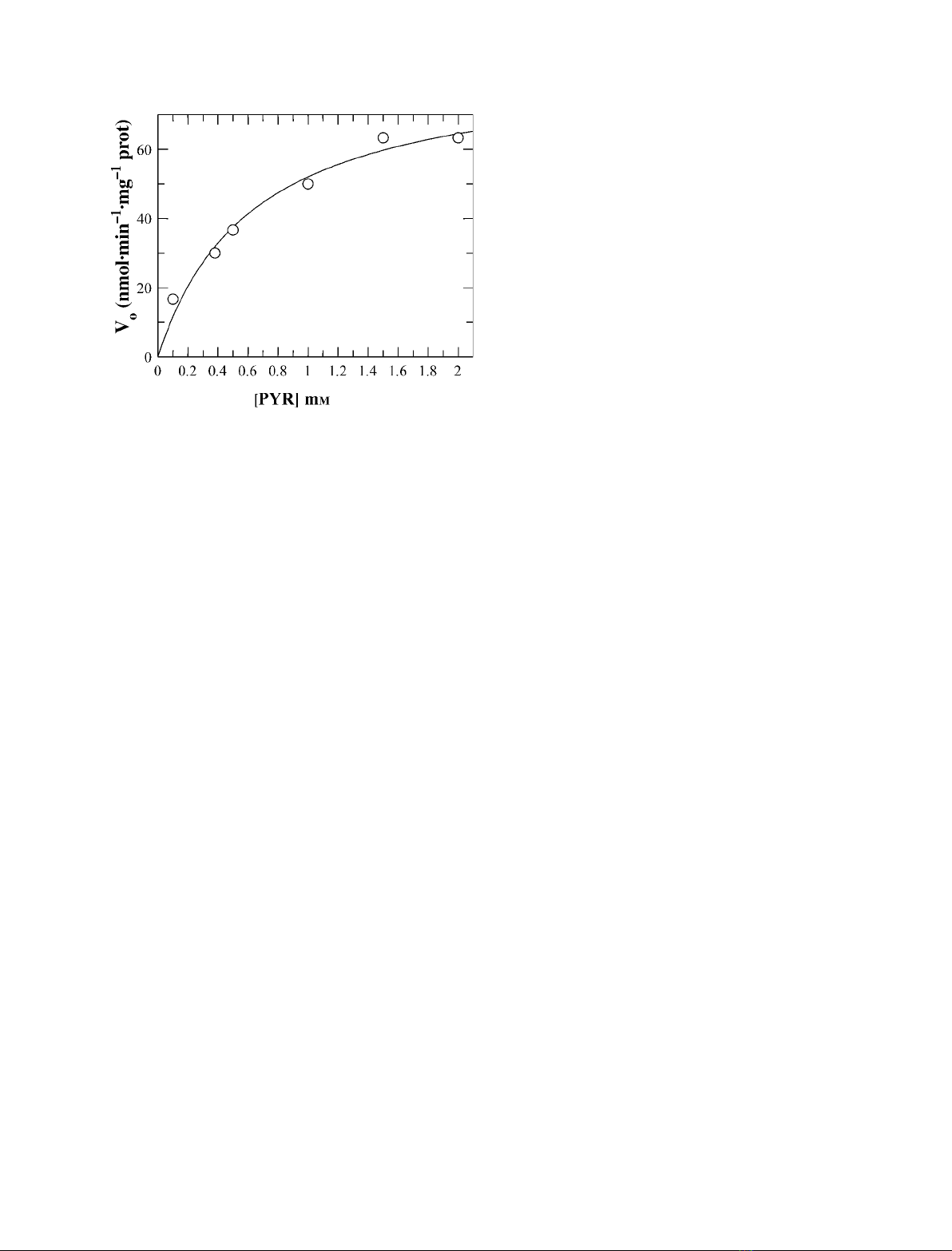
(Fig. 3). Saturation kinetics were found with a K
m
value
of 0.63 ± 0.14 mm; the V
max
value was 85 ± 7 nmolÆ
min
)1
Æmg
)1
sample protein.
Unfortunately, spontaneous oxidation of the NADH
formed during the oxidation of l-lactate prevented
assay with l-lactate and NAD
+
as the substrate pair.
L-Lactate metabolism in mitochondria
Uptake and metabolism of l-lactate was further inves-
tigated in a set of experiments carried out with isolated
coupled PTM. The assumption here is that the mitoch-
ondrial LDH is devoted to oxidation of l-lactate
rather than reduction of pyruvate, as the latter would
be immediately oxidized by the pyruvate dehydroge-
nase complex (K
m
¼0.06 mm[20]). l-Lactate metabo-
lism was monitored by determining the ability of
externally added l-lactate to reduce intramitochondrial
dehydrogenase cofactors. In this case, we resorted to
fluorimetric techniques that have previously been used
to monitor changes in the redox state of pyridine nu-
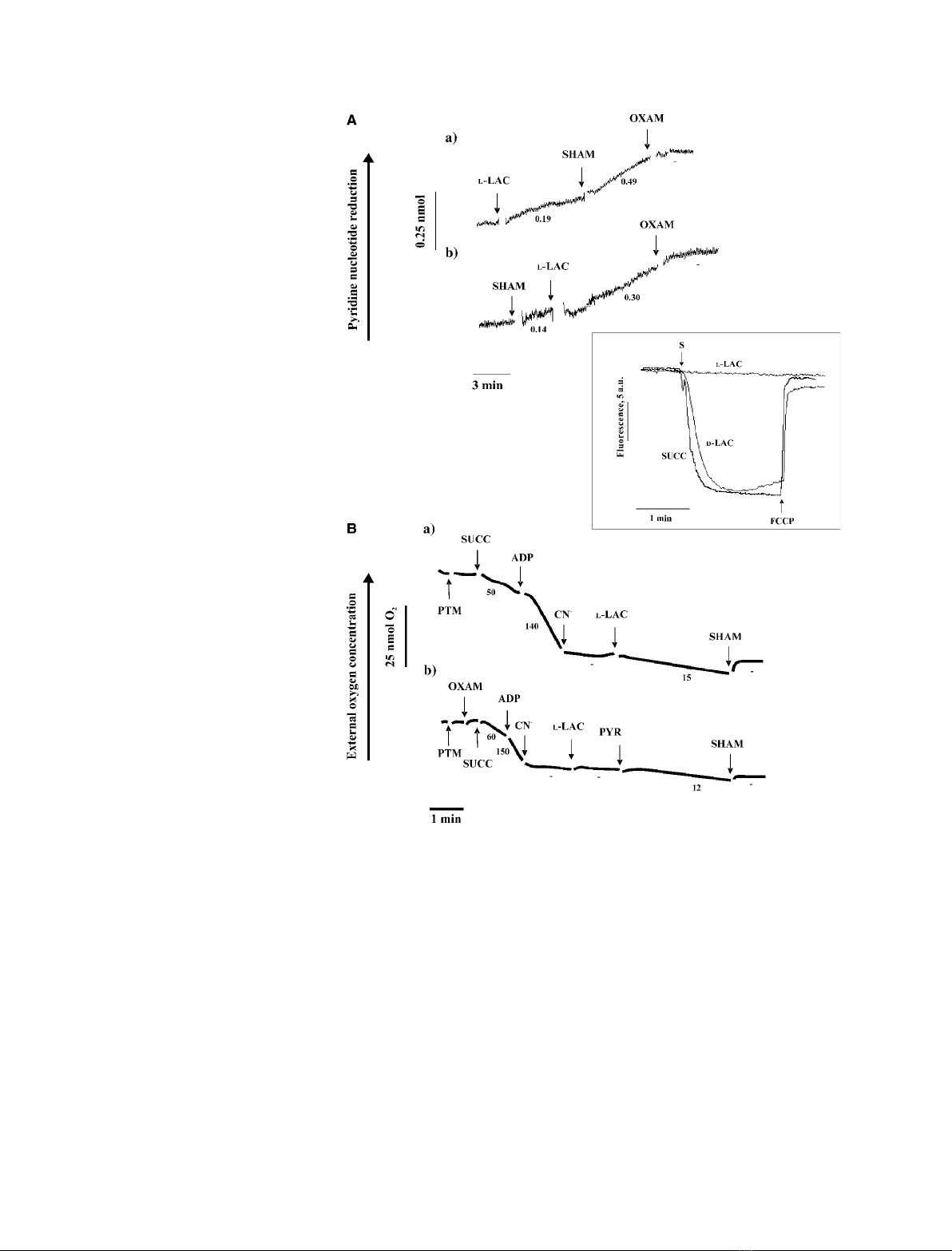
cleotides [21]. Reduction of mitochondrial NAD(P)
+
was found to occur at a rate of 0.19 nmolÆmin
)1
Æmg
)1
protein when l-lactate was added to PTM previously
incubated with or without the uncoupler carbonyl
cyanide p-(trifluoromethoxy)-phenylhydrazone (FCCP)
and then treated with cyanide (CN
–
) (not shown). The
observed rate of reduction was, however, likely to be
underestimated, as the newly formed NAD(P)H would
be rapidly oxidized by the mitochondrial AOX, which
is usually activated by pyruvate, the product of l-lac-
tate metabolism. Hence, we checked whether inhibition
of AOX would cause an increase in the measured rate
of pyridine nucleotide reduction. To achieve this, use
was made of salicyl hydroxamic acid (SHAM, 1 mm),
an AOX inhibitor [22]. Addition of SHAM resulted in
a 150% increase in the measured rate of NAD(P)H
formation (Fig. 4A,a). Consistently, addition of l-lac-
tate to PTM previously incubated with SHAM caused
an increase in the rate of NAD(P)H formation
(Fig. 4A,b). In both cases, the addition of oxamate
(10 mm), an inhibitor of LDHs [23], completely
blocked the increase in fluorescence.
The failure of NADH, newly synthesized during
l-lactate oxidation, to be oxidized in the cytochrome
pathways was confirmed in another experiment (inset
to Fig. 4), in which we checked whether addition of
l-lactate to PTM could produce an increase in the
membrane potential as measured by using safranine O
as a fluorimetric probe. In contrast to succinate
(5 mm) and d-lactate (10 mm), l-lactate (10 mm) failed
to generate a change in electrical membrane potential,
DY. As expected, externally added FCCP (1 lm)
caused membrane potential collapse.
In the same experiment, we investigated, as in
Pastore et al. [24], whether l-lactate itself could activate
AOX, and obtained the results shown in Fig. 4B. In
this case, succinate was added to the mitochondria, fol-
lowed by ADP. Oxygen consumption via the electron
transfer chain was then blocked with CN
–
, and finally
l-lactate was added either in the absence (a) or presence
(b) of oxamate. In the former case, oxygen consump-
tion was restored, but in the latter, l-lactate addition
failed to restore oxygen uptake, thus showing that
l-lactate itself was not responsible for AOX activation.
It is likely that in the absence of oxamate, activation of
AOX was due to the newly formed pyruvate. In a par-
allel experiment, the ability of l-lactate to cause oxygen
uptake by PTM was investigated. We found that addi-
tion of 10 mml-lactate resulted in oxygen uptake at a
rate of 20 nmol O
2
Æmin
)1
Æmg
)1
protein. As expected,
this uptake was not stimulated by 0.2 mmADP, and
was completely prevented following addition of SHAM
(not shown). Control experiments showed that SHAM
did not affect O
2
uptake due to either NADH or succi-
nate in the absence of CN
–
(not shown).
L-Lactate transport in PTM
The experiments reported above raise the question of
how l-lactate produced in the cytosol can cross the
mitochondrial membrane. To gain insight into this,
swelling experiments were carried out as in de Bari
et al. [25]; the results are shown in Fig. 5. PTM
Fig. 3. Assay of LDH activity in PTM solubilized with Triton X-100.
Pyruvate was added at the indicated concentrations to PTM treated
with Triton X-100 (0.2%). The rates (v
o
) of NADH oxidation, calcula-
ted as difference of rate in traces (b) and (a) of Fig. 2C, are
expressed as nmol pyruvate reducedÆmin
)1
Æmg
)1
protein.
L-Lactate metabolism in PTM G. Paventi et al.
1462 FEBS Journal 274 (2007) 1459–1469 ª2007 The Authors Journal compilation ª2007 FEBS
suspended in 0.18 mammonium l-lactate showed
spontaneous swelling, but with a rate and to an extent
significantly lower than those found with ammonium
d-lactate, as judged by statistical analysis of five swell-
ing experiments using Student’s t-test (P<0.02). This
shows that both d-lactate and l-lactate can enter
PTM, but that the uptake is stereospecific. The results
indicate that l-lactate enters mitochondria in a proton-
compensated manner. The metabolite transport para-
digm proposed in Passarella et al. [21] suggests that
net carbon uptake by mitochondria is accompanied by
efflux of newly synthesized compound ⁄s. We wished to
determine whether this applies in the case of l-lactate.
In particular, in the light of the occurrence of an l-lac-
tate ⁄pyruvate shuttle in mammalian mitochondria, the
possible efflux of pyruvate as a result of l-lactate addi-
tion to PTM was investigated (Fig. 6A). The concen-
tration of pyruvate outside PTM was negligible, as
shown by the minimal change in absorbance at
334 nm found when commercial LDH was added
along with the NADH to complete the pyruvate-
detecting system (for details, see Experimental proce-
dures). On the other hand, in the presence of l-lactate
(10 mm), the absorbance at 334 nm decreased rapidly,
which is indicative of the appearance of pyruvate in
the extramitochondrial phase. This can be explained
on the basis that the l-lactate imported into the mito-
chondria forms pyruvate via the mitochondrial LDH,
Fig. 4. Effect of L-lactate addition to PTM.
Change in the redox state of pyridine nucle-
otides (A), failure to cause membrane poten-
tial generation (inset), and activation of AOX
(B). (A) PTM (0.2 mg protein) were incuba-
ted in 2 mL of the standard medium (see
Experimental procedures), and the fluores-
cence (k
ex
334 nm, k
em
456 nm) was con-
tinuously monitored. At the times indicated
by the arrows, L-lactate (10 mm), SHAM
(1 mM), and oxamate (OXAM, 10 mM) were
added. The numbers alongside the traces
refer to the rate of reduction of NAD(P)
+
in
nmolÆmin
)1
Æmg
)1
protein. Inset: PTM
(0.2 mg of protein) were incubated in 2 mL
of the standard medium in the presence of
2.5 lMsafranin, and fluorescence
(k
ex
520 nm, k
em
570 nm), measured as
arbitrary units (a.u.), was continuously
monitored. Where indicated by S, L-lactate
(L-LAC, 10 mM), D-lactate (D-LAC, 10 mM)or
succinate (5 mM) were added separately;
where indicated, FCCP (1 lM) was added.
(B) PTM (0.2 mg protein) were suspended
at 25 C in 1 mL of respiratory medium, and
the amount of residual oxygen was meas-
ured as a function of time. Where indicated,
the following additions were made: succi-
nate (SUCC, 5 mM), oxamate (10 mM), ADP
(0.2 mM), cyanide (CN
–
,1mM), L-lactate
(L-LAC, 10 mM), pyruvate (PYR, 5 mM), and
SHAM (1 mM). Numbers along the curves
are rates of oxygen uptake expressed as
nmol O
2
Æmin
)1
Æmg
)1
mitochondrial protein.
G. Paventi et al.L-Lactate metabolism in PTM
FEBS Journal 274 (2007) 1459–1469 ª2007 The Authors Journal compilation ª2007 FEBS 1463


























