
Applied Catalysis, 38 (1988) 143-155 zyxwvutsrqponmlkjihgfedcbaZYXWVUTSRQPONMLKJIHGFEDCBA
Elsevier Science Publishers B.V., Amsterdam - Printed in The Netherlands
143 zyxwvutsrqponmlkjihgfedcbaZYXWVUTSRQPONMLKJIHGFEDCBA
Catalytic Cracking of Heavy Oil over Catalysts
Containing Different Types of Zeolite Y in Active
and Inactive Matrices
J.-E. OTTERSTEDT*, YAN-MING ZHU’ and J. STERTE
Department of Engineering Chemistry I, Chalmers University of Technology, 412 96 Giiteborg
(Sweden)
(Received 3 July 1987, accepted 22 September 1987)
ABSTRACT
The effects of addition of alumina to the matrices of cracking catalysts containing different
types of zeolite Y, on their cracking performance, were investigated using a micro activity test and
two different feed oils.
For the heavier feed oil, the alumina addition resulted in a higher conversion at the same catalyst
to oil ratio independent of the type of zeolite. This higher conversion was accompanied by a greater
selectivity for coke and a lower selectivity for gasoline. For the lighter feed oil the effect of alumina
addition on the total conversion was much less pronounced while the effects on the selectivity
were similar to those observed using the heavier feed.
The performance of the catalysts in a commercial fluid catalytic cracking unit is discussed in
view of their coke forming tendencies and the heat balance of the cracker.
INTRODUCTION zyxwvutsrqponmlkjihgfedcbaZYXWVUTSRQPONMLKJIHGFEDCBA
In the past decade there has been a trend towards using heavier feedstocks
in fluid catalytic cracking (FCC ) . The composition of these heavy cuts have
caused problems of different kinds. The pore structure of the zeolite in zeolite
catalysts is too fine to allow fast diffusion of the large molecules present in
these fractions. Typically these feeds have high contents of metals, such as
vanadium and nickel, aromatics and compounds containing heteroatoms of
nitrogen, sulfur and oxygen. The effect of vanadium is primarily a decrease in
catalyst activity while the major effect of nickel is a selectivity change reflected
in increased coke and gas yields [ 11. The high-boiling aromatics act as coke
precursors and the increased amount of coke on the catalyst, in addition to
rapidly deactivating the catalyst, also causes problems with the heat balance
*Visiting Scholar from Yanshan Petro-Chemical Company, Peoples Republic of China.
0166-9834/ 88/ $03.50 0 1988 Elsevier Science Publishers B.V.

144 zyxwvutsrqponmlkjihgfedcbaZYXWVUTSRQPONMLKJIHGFEDCBA
of the FCC unit since excess heat is produced in the regenerator when the coke
is burned off.
The selectivity problem caused by nickel contaminants can be reasonably
handled by adding nickel passivators, usually containing antimony, to the feed
[ 21. Although passivators have been reported to decrease the deactivation ef-
fects of vanadium, a more promising way to handle this problem is to introduce
vanadium “ traps” into the catalyst, which prevent the vanadium from migrat-
ing to, and destroying the zeolite [ 31. In order to meet the problem of cracking
large molecules, all major manufacturers of FCC catalysts have developed cat-
alysts containing active matrices, i.e. matrices showing a considerable cracking
activity even without the zeolite component. The idea behind this is that an
initial cracking of the molecules, too large to penetrate the zeolite structure,
occurs on the matrix surface, making a cosecutive cracking of the intermediate
species in the zeolite pores possible.
One material commonly used in commercial catalysts and reported to func-
tion both as a vanadium trap and as the active component in active matrices
is alumina [ 3 ] .
This paper reports on a systematic study of the effects of alumina addition
to the matrix of cracking catalysts, containing different types of zeolite Y, on
the cracking performance of these catalysts when cracking conventional and
heavy feed oils. Catalysts containing rare earth exchanged zeolite Y (REY) ,
calcined REY zyxwvutsrqponmlkjihgfedcbaZYXWVUTSRQPONMLKJIHGFEDCBA(CREY) , ultrastable zeolite Y (USY ) and rare earth exchanged
USY (REUSY) in kaolin-binder and in kaolin-alumina-binder matrices were
prepared and investigated for catalytic cracking using a micro activity test.
EXPERIMENTAL zyxwvutsrqponmlkjihgfedcbaZYXWVUTSRQPONMLKJIHGFEDCBA
Preparation of zeolites
A sample of rare earth exchanged zeolite Y was prepared by cation exchange
of a NaY (Katalistiks) with a solution containing a mixture of rare earth chlo-
rides (Rhone Poulenc) . The sample was exchanged three times using a solu-
tion containing 9 wt% REC& kept at 95°C for 1.0 h. After the third exchange
the sample was washed until chloride free by repetitive reslurrying in distilled
water followed by separation by filtration. The REY was dried at 120” C for 4.5
h. The CREY was prepared as follows, A sample of NaY was first subjected to
two ion exchanges with REC& as described above. The resulting zeolite was
then dried at 120°C for 4.5 h and calcined at 500°C for 2.5 h. The calcined
zeolite was then subjected to a third RE-exchange and finally to a NH,+ -ex-
change using a solution containing 20 wt% ammonium sulfate. This exchange
was also performed at 95 o C using a contact time of 1.0 h. The resulting CREY
was washed, separated and dried in the same manner as the REY.
USY was prepared by subjecting a sample of NH,Y (Katalistiks) to repet-
145
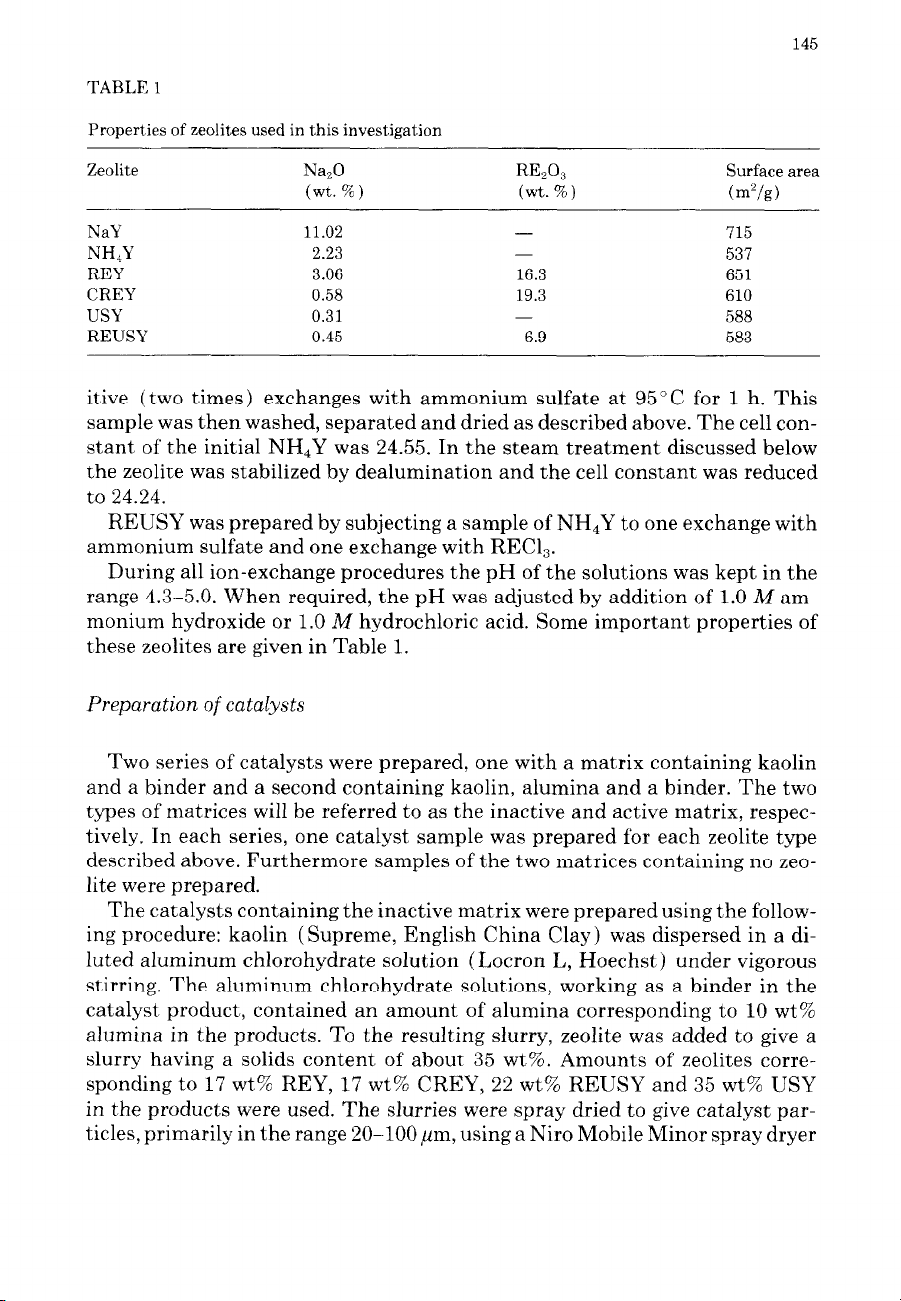
TABLE 1
Properties of zeolites used in this investigation
Zeolite
NaY
NHIY
REY
CREY
USY
REUSY
Na,O
(wt. %)
11.02
2.23
3.06
0.58
0.31
0.45
R&G
(wt. %)
-
16.3
19.3
6.9
Surface area
(m”/g)
715
537
651
610
588
583
itive (two times) exchanges with ammonium sulfate at 95 o C for 1 h. This
sample was then washed, separated and dried as described above. The cell con-
stant of the initial NH,Y was 24.55. In the steam treatment discussed below
the zeolite was stabilized by dealumination and the cell constant was reduced
to 24.24.
REUSY was prepared by subjecting a sample of NH,Y to one exchange with
ammonium sulfate and one exchange with RECl,.
During all ion-exchange procedures the pH of the solutions was kept in the
range 4.3-5.0. When required, the pH was adjusted by addition of 1.0 zyxwvutsrqponmlkjihgfedcbaZYXWVUTSRQPONMLKJIHGFEDCBAM am-
monium hydroxide or 1.0 M hydrochloric acid. Some important properties of
these zeolites are given in Table 1.
Preparation of catalysts
Two series of catalysts were prepared, one with a matrix containing kaolin
and a binder and a second containing kaolin, alumina and a binder. The two
types of matrices will be referred to as the inactive and active matrix, respec-
tively. In each series, one catalyst sample was prepared for each zeolite type
described above. Furthermore samples of the two matrices containing no zeo-
lite were prepared.
The catalysts containing the inactive matrix were prepared using the follow-
ing procedure: kaolin (Supreme, English China Clay) was dispersed in a di-
luted aluminum chlorohydrate solution (Locron I+ Hoechst) under vigorous
stirring. The aluminum chlorohydrate solutions, working as a binder in the
catalyst product, contained an amount of alumina corresponding to 10 wt%
alumina in the products. To the resulting slurry, zeolite was added to give a
slurry having a solids content of about 35 wt%. Amounts of zeolites corre-
sponding to 17 wt% REY, 17 wt% CREY, 22 wt% REUSY and 35 wt% USY
in the products were used. The slurries were spray dried to give catalyst par-
ticles, primarily in the range 20-100 pm, using a Niro Mobile Minor spray dryer
146
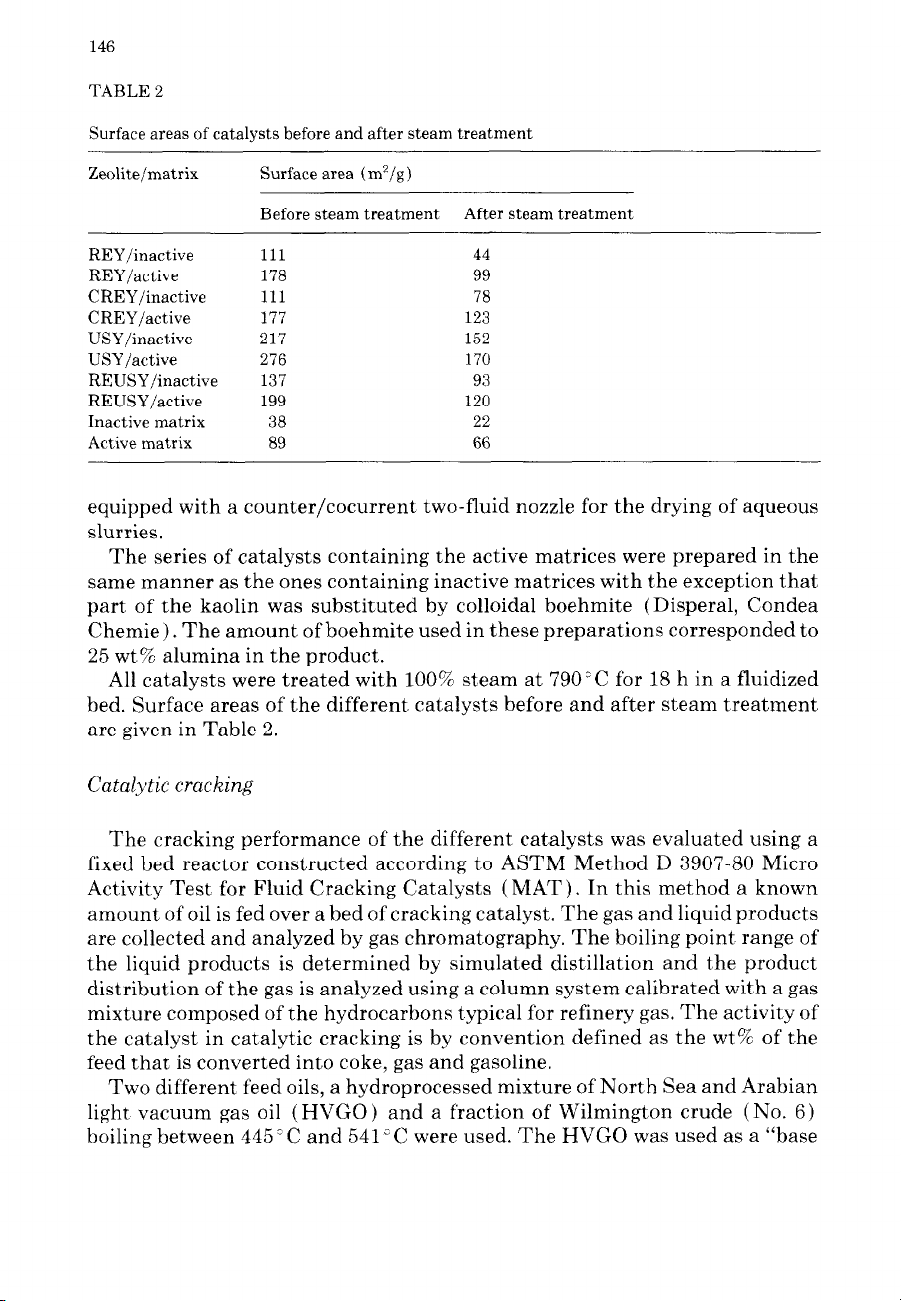
TABLE 2
Surface areas of catalysts before and after steam treatment
Zeolite/matrix Surface area (m’/g)
Before steam treatment After steam treatment
REY/inactive 111 44
REY/active 178 99
CREY/inactive 111 78
CREY/active 177 123
USY/inactive 217 152
USY/active 276 170
REUSY/inactive 137 93
REUSY/active 199 120
Inactive matrix 38 22
Active matrix 89 66
equipped with a counter/cocurrent two-fluid nozzle for the drying of aqueous
slurries.
The series of catalysts containing the active matrices were prepared in the
same manner as the ones containing inactive matrices with the exception that
part of the kaolin was substituted by colloidal boehmite (Disperal, Condea zyxwvutsrqponmlkjihgfedcbaZYXWVUTSRQPONMLKJIHGFEDCBA
Chemie) . The amount of boehmite used in these preparations corresponded to
25 wt’% alumina in the product.
All catalysts were treated with 100% steam at 790°C for 18 h in a fluidized
bed. Surface areas of the different catalysts before and after steam treatment
are given in Table 2. zyxwvutsrqponmlkjihgfedcbaZYXWVUTSRQPONMLKJIHGFEDCBA
Catalytic cracking
The cracking performance of the different catalysts was evaluated using a
fixed bed reactor constructed according to ASTM Method D 3907-80 Micro
Activity Test for Fluid Cracking Catalysts (MAT ) . In this method a known
amount of oil is fed over a bed of cracking catalyst. The gas and liquid products
are collected and analyzed by gas chromatography. The boiling point range of
the liquid products is determined by simulated distillation and the product
distribution of the gas is analyzed using a column system calibrated with a gas
mixture composed of the hydrocarbons typical for refinery gas. The activity of
the catalyst in catalytic cracking is by convention defined as the wt% of the
feed that is converted into coke, gas and gasoline.
Two different feed oils, a hydroprocessed mixture of North Sea and Arabian
light vacuum gas oil (HVGO ) and a fraction of Wilmington crude (No. 6)
boiling between 445 ‘C and zyxwvutsrqponmlkjihgfedcbaZYXWVUTSRQPONMLKJIHGFEDCBA541 3 C were used. The HVGO was used as a “base
147
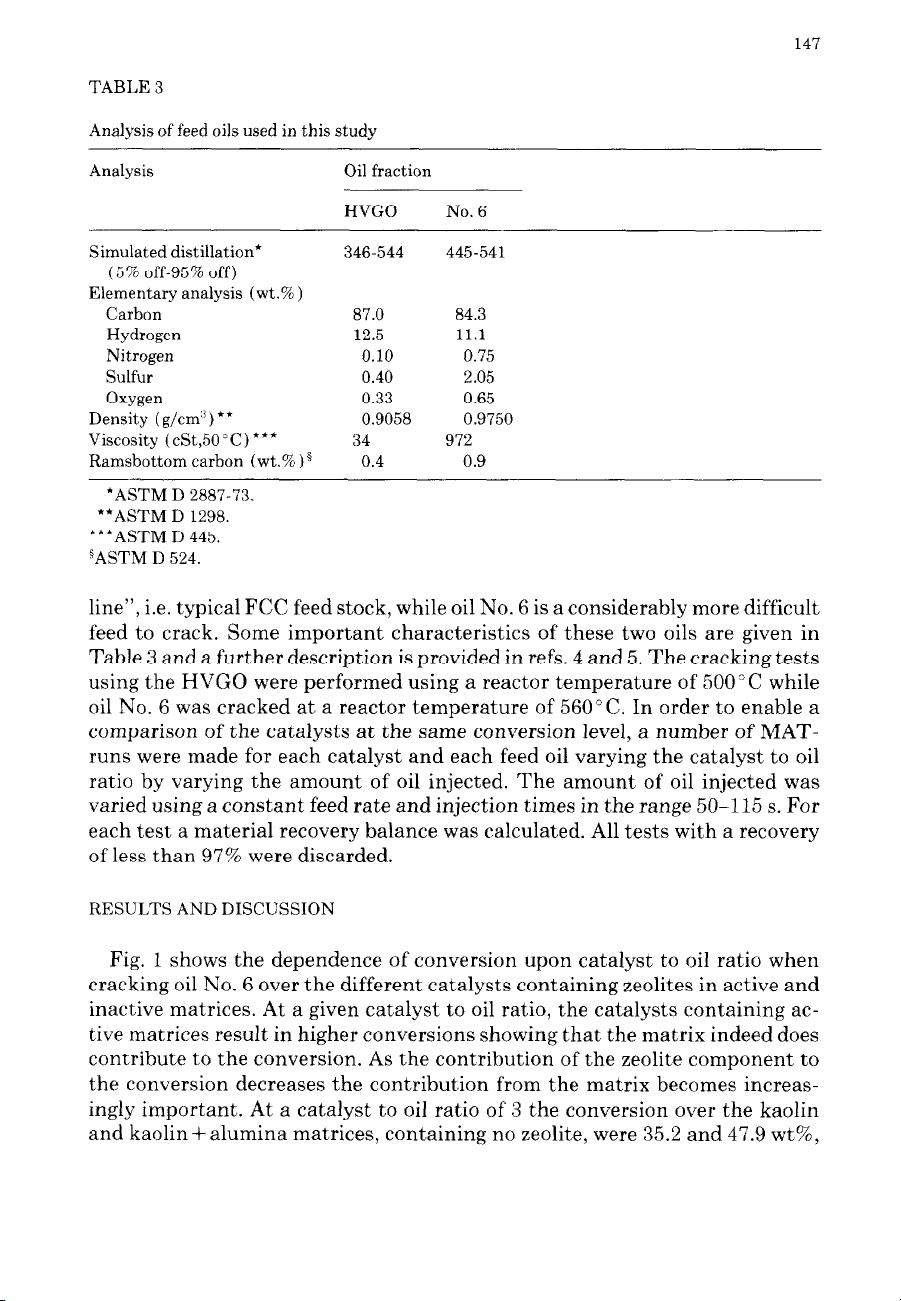
TABLE 3
Analysis of feed oils used in this study
Analysis Oil fraction
HVGO No. 6
Simulated distillation*
(5% off-95% off)
Elementary analysis (wt.% )
Carbon
Hydrogen
Nitrogen
Sulfur
Oxygen
Density (g/cm”) **
Viscosity (cSt,SO’C) ***
Ramsbottom carbon (wt.% ) g
346-544 445-541
87.0 84.3
12.5 11.1
0.10 0.75
0.40 2.05
0.33 0.65
0.9058 0.9750
34 972
0.4 0.9
*ASTM D 2887-73.
**ASTM D 1298.
***ASTM D 445.
“ASTM D 524.
line”, i.e. typical FCC feed stock, while oil No. 6 is a considerably more difficult
feed to crack. Some important characteristics of these two oils are given in
Table 3 and a further description is provided in refs. 4 and 5. The cracking tests
using the HVGO were performed using a reactor temperature of 500°C while
oil No. 6 was cracked at a reactor temperature of 560°C. In order to enable a
comparison of the catalysts at the same conversion level, a number of MAT-
runs were made for each catalyst and each feed oil varying the catalyst to oil
ratio by varying the amount of oil injected. The amount of oil injected was
varied using a constant feed rate and injection times in the range 50-115 s. For
each test a material recovery balance was calculated. All tests with a recovery
of less than 97% were discarded.
RESULTS AND DISCUSSION
Fig. 1 shows the dependence of conversion upon catalyst to oil ratio when
cracking oil No. 6 over the different catalysts containing zeolites in active and
inactive matrices. At a given catalyst to oil ratio, the catalysts containing ac-
tive matrices result in higher conversions showing that the matrix indeed does
contribute to the conversion. As the contribution of the zeolite component to
the conversion decreases the contribution from the matrix becomes increas-
ingly important. At a catalyst to oil ratio of 3 the conversion over the kaolin
and kaolin+alumina matrices, containing no zeolite, were 35.2 and 47.9 wt%,


























