
TNU Journal of Science and Technology
230(05): 263 - 270
http://jst.tnu.edu.vn 263 Email: jst@tnu.edu.vn
IMPACT OF SPERM PREPARATION TECHNIQUES ON
BOER GOAT SPERM QUALITY BEFORE CRYOPRESERVATION
Tran Thi Thanh Khuong*, Nguyen Lam Khanh Duy, Nguyen Nhat Tan, Ha Thi Ngoc Trang,
Tran Van Be Nam
Can Tho University
ARTICLE INFO
ABSTRACT
Received:
07/10/2024
Sperm preparation is a technique to select good quality sperm for sperm
freezing and artificial insemination. This study investigated the effects of
swim-up time and concentration gradient centrifugal force on the quality of
Boer goat sperm. Two Boer goats were selected for semen collection using
an artificial vagina. Then, the semen was selected by two methods: swim-
up with time points (30, 45 and 60 minutes) and centrifugation of gradient
medium with centrifugal force (250g, 300g, 350g and 400g). Then, the
sperm quality assessment indicators were performed. The results of the
study showed that swim-up time for 30 minutes significantly improved the
quality of goat sperm after collection with the mean viability, overall
motility, progressive motility, membrane and acrosome integrity being
92.81%, 84.89%, 53.35%, 62.93% and 97.90%, respectively (p<0.05).
Centrifugal force at 350g results in the selection of optimal goat sperm
quality with the mean viability, overall motility, progressive motility,
membrane and acrosome integrity being 88.54%, 80.21%, 55.46%,
70.61%, and 59.55% respectively (p<0.05). In conclusion, swim-up time in
30 minutes and concentration gradient centrifugal force in 350g were the
optimal choices for the Boer goat sperm selection process. The study aimed
to emphasize the optimization of sperm preparation procedures to select the
best quality goat sperm for cryopreservation and artificial insemination,
which is of great significance for animal breeding and conservation efforts.
Revised:
06/02/2025
Published:
07/02/2025
KEYWORDS
Boer goat
Concentration gradient
centrifugal
Insemination
Sperm
Swim-up
ẢNH HƯỞNG CỦA KỸ THUẬT CHUẨN BỊ TINH TRÙNG ĐẾN
CHẤT LƯỢNG TINH TRÙNG DÊ BOER TRƯỚC KHI ĐÔNG LẠNH
Trần Thị Thanh Khương*, Nguyễn Lâm Khánh Duy, Nguyễn Nhật Tân, Hà Thị Ngọc Trang,
Trần Văn Bé Nam
Trường Đại học Cần Thơ
THÔNG TIN BÀI BÁO
TÓM TẮT
Ngày nhận bài:
07/10/2024
Chuẩn bị tinh trùng là một kỹ thuật để lựa chọn tinh trùng chất lượng tốt cho
việc đông lạnh tinh trùng và thụ tinh nhân tạo. Nghiên cứu này đã tìm hiểu
ảnh hưởng của thời gian bơi lên và lực ly tâm nồng độ lên chất lượng tinh
trùng dê Boer. Nghiên cứu được tiến hành trên hai con dê Boer đực, mẫu
tinh dịch được thu nhận bằng cách sử dụng âm đạo nhân tạo. Sau đó, tinh
dịch được lựa chọn bằng hai phương pháp: bơi lên (30, 45 và 60 phút) và ly
tâm gradient với lực (250 g, 300 g, 350 g và 400 g). Kết quả nghiên cứu cho
thấy thời gian bơi lên trong 30 phút đã cải thiện đáng kể chất lượng tinh
trùng dê với tỷ lệ sống, di động tổng số, di động tiến tới, toàn vẹn của màng
và acrosome lần lượt là 92,81%, 84,89%, 53,35%, 62,93% và 97,90% (p <
0,05). Lực ly tâm ở 350 g giúp tinh trùng dê đạt chất lượng tối ưu với tỷ lệ
sống, di động tổng số, di động tiến tới, toàn vẹn của màng và acrosome lần
lượt là 88,54%, 80,21%, 55,46%, 70,61% và 59,55% (p <0,05). Thời gian
bơi lên trong 30 phút và lực ly tâm nồng độ gradient ở 350 g là những lựa
chọn tối ưu cho quá trình lựa chọn tinh trùng dê Boer. Nghiên cứu nhằm
nhấn mạnh việc tối ưu hóa các quy trình chuẩn bị tinh trùng dê, có ý nghĩa to
lớn đối với các nỗ lực nhân giống và bảo tồn động vật.
Ngày hoàn thiện:
06/02/2025
Ngày đăng:
07/02/2025
TỪ KHÓA
Dê Boer
Ly tâm gradient nồng độ
Thụ tinh
Tinh trùng
Swim-up
DOI: https://doi.org/10.34238/tnu-jst.11259
* Corresponding author. Email: tttkhuong@ctu.edu.vn

TNU Journal of Science and Technology
230(05): 263 - 270
http://jst.tnu.edu.vn 264 Email: jst@tnu.edu.vn
1. Introdution
The goat farming and goat meat supply industry in Vietnam are growing rapidly, with the
Mekong Delta alone accounting for 15% of the total herd of country [1]. The Boer goat breed
specializes in meat, is easy to raise, has fewer diseases, and is highly productive [2]. The
application of artificial insemination brings high reproductive efficiency [3], [4]. Sperm freezing
is a widely used as a method of sperm preservation in assisted reproductive technology. Various
freezing methods have been developed to minimize damage to sperm cells [5]. However, to
improve sperm quality after thawing, sperm preparation is a method to select the best sperm
while still maintaining high mobility and normal morphology [6], and at the same time select
sperm with good DNA quality, eliminating leukocytes and bacteria [7]. Two effective and
popular methods are swim-up and concentration gradient centrifugation (CGC) [8].
Studies comparing swim-up and concentration gradient methods have shown mixed results.
Swim-up was found to significantly reduce sperm DNA fragmentation rates in both unexplained
and mild male factor infertility cases [9]. However, another study reported that the density
gradient technique was superior in separating normal, motile spermatozoa with healthy DNA
compared to swim-up [10]. Both methods were effective in eliminating sperm with DNA damage
in normozoospermic, oligozoospermic, and teratozoospermic samples [11]. For semen samples
with low sperm concentration (<20 million/ml), the Percoll gradient technique selected sperm
superior to those separated by swim-up in terms of progressive motility, ATP content, energy
charge index, and morphology [12]. The swim-up technique consistently demonstrated superior
ability to reduce sperm DNA fragmentation compared to CGC [13]. Swim-up also yielded higher
sperm progressive motility than DGC [14]. However, CGC showed better results in reducing
abnormal chromatin structure [13] and provided a higher recovery rate of progressive motile
sperm [14]. Both methods significantly improved sperm motility and morphology compared to
unprocessed samples [9], [10]. Interestingly, neither method significantly affects the percentage
of sperm with normal sex chromosomes [13]. There have been many studies on the swim-up
method in horses and humans [15] - [17] and the gradient concentration centrifugation method in
goats and humans [18], [19]. The aim of this study is to determine the optimal swim-up time and
gradient centrifugation force to select good quality sperm for sperm freezing and artificial
insemination.
2. Materials and methods
2.1. Chemicals
The chemicals used in this study included Citric Acid (Sigma, USA), Eosin (Himedia, India),
Fructose (Sigma, USA), Glucose (Sigma, USA), NaHCO3 (Thermo Fisher Scientific, USA),
Nigrosin (Himedia, India), Spermgrad 45% (Vitrolife, San Diego, CA, USA), Spermgrad 90%
(Vitrolife, San Diego, CA, USA). These chemicals are necessary for preparing semen dilution
medium, performing sperm quality assessments, and conduct experiments.
2.2. Animals
Two Boer goats over 12 months old, raised at the experimental animal farm of the Stem Cell
Laboratory, Can Tho University. The diet of goats was formulated to meet the nutritional needs
of adult male goats [20]. This study was conducted with ethical approval for the procedures of
animal care, housing and semen collection according to the Code of Ethics in Animal
Experiments of Can Tho University (CTU-AEC24013) [21].
2.3. Experiment design
Sperm collection was performed using an artificial vagina warmed to 40–42 °C.

TNU Journal of Science and Technology
230(05): 263 - 270
http://jst.tnu.edu.vn 265 Email: jst@tnu.edu.vn
Experiment 1: The swim-up method based on Magdanz et al. [22] was used. In a 15-mL
falcon tube, 1 mL of Tris Citrate Glucose (TCG) medium was added, and then 0.5 mL of fresh
semen was gently injected along the wall of the tube to form two layers. The falcon tube was
tilted at a 45°. The swim-up process was conducted at 37°C for three time points: 30 min, 45 min,
and 60 min. The supernatant containing healthy sperm was aspirated and examined quality.
Experiment 2: The concentration gradient centrifugation method, based on Balachandran et al.
[23], was used. In a 15-mL falcon tube, 1 mL of the upper Spermgrad medium (45%), followed
by 1 mL of the lower Spermgrad medium (90%) was carefully placed at the bottom of the tube,
and then 0.5 mL of fresh semen was gently injected along the wall of the tube to form three
layers. The falcon tube was centrifuged for 15 min at 4 rotation forces: 250 g, 300 g, 350 g, and
400 g. The sediment was transferred to a new tube containing 3 mL of Tris Citrate Glucose
(TCG) medium. The centrifugation step was repeated. The second centrifuged sediment was
washed with 0.5 mL of TCG medium and the sperm quality was examined.
2.4. Assessment of Sperm concentration
Following the loading of 9μL of the sample, the counting chamber was given four minutes to
acclimate to ambient temperature. A minimum of 200 intact spermatozoa were counted each
counting chamber under a 40X magnification microscope. The World Health Organization
criteria [24] were followed in order to calculate the sperm count.
2.5. Assessment of Sperm motility
Two wet mounts were made on a counting chamber for each sample. Spermatozoa motility
was assessed by classifying them into three groups: immotility, non-immotility, and progressive
motility. Two distinct wet mounts underwent two counts, and the outcomes of the two mounts
were compared, and the average was determined [25].
2.6. Assessment of Sperm viability
Sperm viability was assessed using the Eosin-Nigrosin method [26]. Viable spermatozoa were
identified by their white appearance or partial red or dark pink staining in the neck region, while the
remaining head portion remained unstained. In contrast, dead spermatozoa exhibited a reddish or
dark pink coloration in the head region. The rate of viability spermatozoa was calculated based on
the observed counts.
2.7. Assessment of Sperm membrane integrity
The Hypo-Osmotic Swelling Test (HOS Test) was employed to assess the sample. An
Eppendorf tube containing 20 µL of semen sample and 80 µL of HOS solution was placed in a
37°C incubator. After 40 minutes of incubation, a 10 µL portion of the mixture was placed on a
glass slide for microscopic examination. Spermatozoa with intact membranes exhibited swelling
in the tail region, whereas those with compromised membranes showed no swelling [27].
2.8. Assessing sperm acrosome integrity
The acrosome integrity of spermatozoa was analyzed by Giemsa staining [28] and observed
using an Eclipse Si microscope (Nikon) with 40X magnification. Sperm with normal acrosomes
will have a head region that will stain with Giemsa dye (purple), whereas sperm with abnormal
acrosomes will have a head region that will not stain with dye. The percentage of spermatozoa
with acrosome integrity was determined by counting the number of spermatozoa with dye
staining over the total number of spermatozoa counted. The percentage of spermatozoa with
acrosome integrity was determined by counting the number of spermatozoa with dye staining
over the total number of spermatozoa counted.
2.9. Statistical analysis
TNU Journal of Science and Technology
230(05): 263 - 270
http://jst.tnu.edu.vn 266 Email: jst@tnu.edu.vn
R 4.3.1 was used along with Excel to analyze the data. The influence of cysteine concentration
was the primary factor investigated. The data were analyzed using a Linear Mixed Model
ANOVA, and then mean comparisons between treatments were performed using the Turkey
method in the R 4.3.1 software. The mean ± standard error (SE) of the results was displayed. A
high degree of confidence in the acquired data was indicated by the statistical significance, which
was established at p < 0.05. The R 4.3.1 software was used to create the graphs
3. Results and Discussion
3.1. Semen quality after sample collection
Table 1 shows the results of the Boer goat sperm quality assessment after collection. All
criterias within the normal value and consistent with the study of Nguyen et al. [25]. From the
quality assessment results, it can be seen that the goat sperm sample is homogeneous and of
sufficient quality for further experiments.
Table 1. Results of fresh semen quality assessment (Mean ± Standard error, N=8)
Volume
0.69 ± 0.01 mL
pH
6.98 ± 0.03
Concentration
2.59 ± 0.03 (×109 cell/mL)
Overall motility
64.71 ± 0.23 %
Progressive motility
42.79 ± 0.42 %
Viability
73.54 ± 0.74 %
Membrane integrity
68.32 ± 0.81 %
Acrosome integrity
86.47 ± 0.59 %
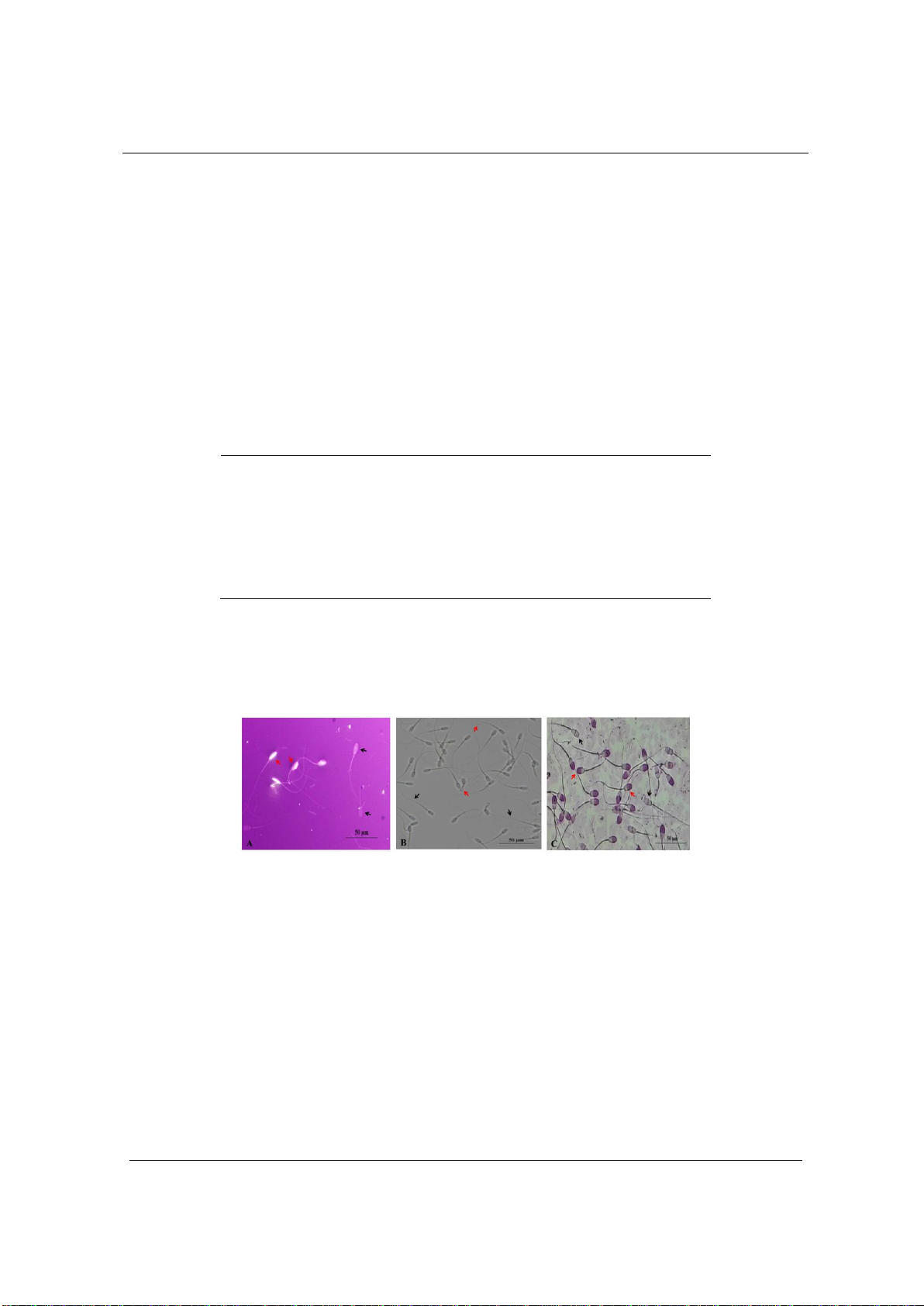
Figure 1 presents the results of the assessment of sperm viability, membrane integrity and
acrosome. In Figure 1.A, live sperm do not take up the dye (red arrow), dead sperm take up the
dye (black arrow). In Figure 1.B, sperm with intact cell membranes show tail curling reaction
(red arrow), sperm with damaged cell membranes do not have reaction (black arrow). In Figure
1.C, sperm with normal acrosomes show the acrosome part taking up the dye (red arrow), sperm
with abnormal acrosomes show the acrosome part not taking up the dye (black arrow).
Figure 1. Sperm quality test results
A - Sperm stained with eosin-nigrosin. B - Sperm tested for HOS. C - Sperm stained with acrosome.
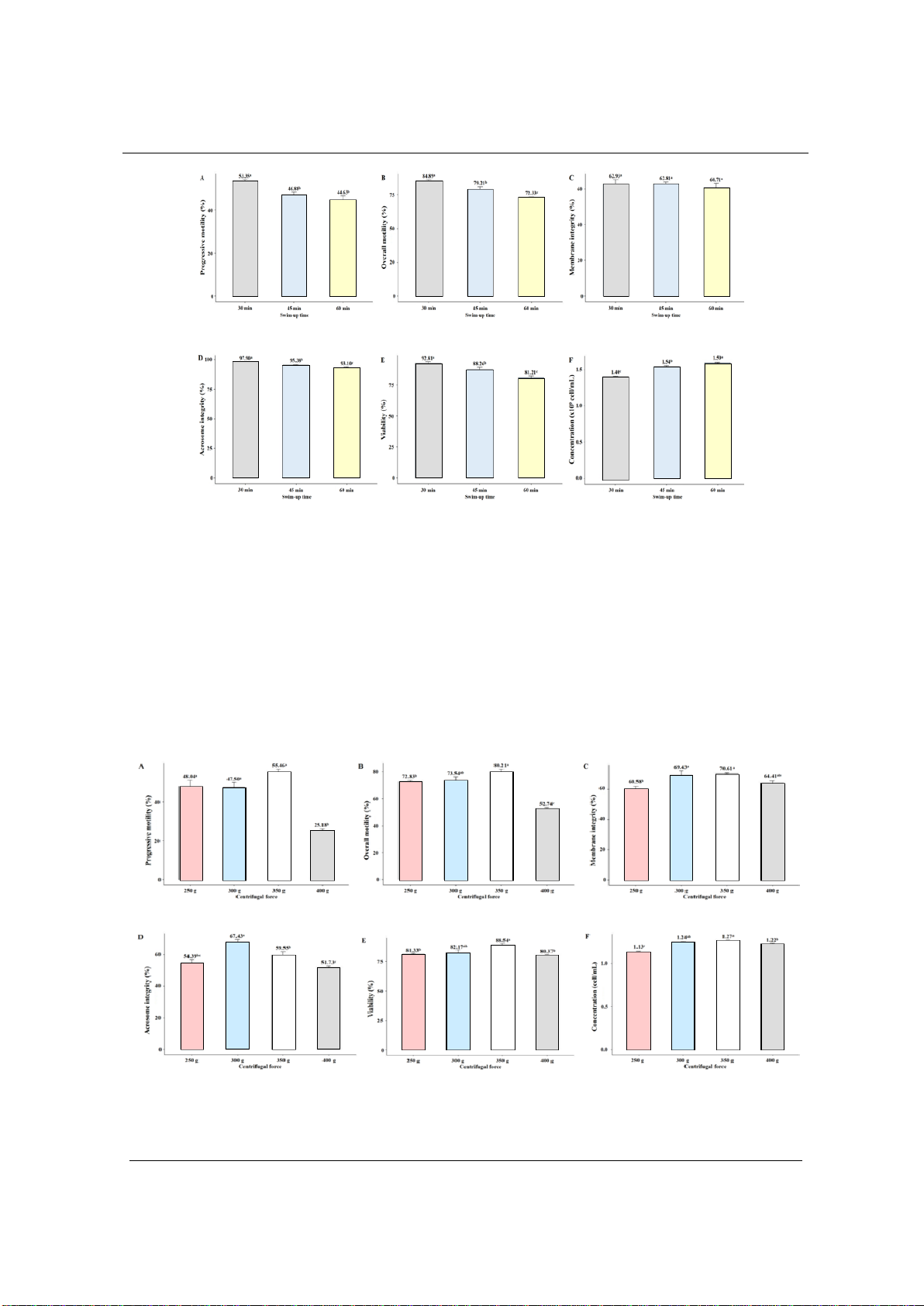
3.2. Effect of swim-up time on sperm quality
The results in Figure 2 show the changes in goat sperm quality after washing by swim-up
method at three different times: 30, 45 and 60 minutes. After 30 minutes of swim-up, the sperm
concentration is 1.40×109 cells/mL and after 45 minutes it is 1.54×109 cells/mL, the difference is
statistically significant (p < 0.05). The sperm overall motility is 84.89% after 30 minutes, 79.21%
after 45 minutes and 72.33% after 60 minutes of swim-up. The sperm progressive motility is
53.35% after 30 minutes of swim-up, after 45 minutes and 60 minutes it is 46.88% and 44.63%,
respectively. The viability rate of goat sperm is 92.81% after 30 min, 88.26% after 45 min, and
81.21% after 60 min of swim-up. The sperm acrosome integrity is 97.90% after 30 min, 95.38%
after 45 min, and 93.10% after 60 min of swim-up. Regarding the integrity of goat sperm
membrane obtained in the 3 experiments of 30, 45, and 60 min, the results are 62.93%, 62.81%,
and 60.71%, respectively, the difference is not statistically significant (p > 0.05).
TNU Journal of Science and Technology
230(05): 263 - 270
http://jst.tnu.edu.vn 267 Email: jst@tnu.edu.vn
Figure 2. Effect of swim-up time on boer goat sperm quality
A- Progressive motility, B- Overall motility, C- Membrane integrity, D- Acrosome integrity, E-Viability
and F- Concentration. Data are expressed as mean values (n=8). a,b,cValues in each criteria with different
superscripts are statistically significantly different; p < 0.05
3.3. Effect of gradient centrifugal force on sperm quality
Figure 3 shows the results after CGC at four different centrifugation forces of 250g, 300g,
350g and 400g. When centrifuged at 350g, the concentration (1.27×109 cells/mL), overall
motility (80.21%), progressive motility (55.46%), membrane integrity (70.61%) and viability
(88.54%) of goat sperm reach the highest rates, but the different indices are not significant when
centrifuged at 300g (P>0.05). However, for the acrosome integrity rate, the sperm sample
centrifuged at 300g gives the best result with 67.43%, the data are significantly different from the
remaining treatments (P<0.05).
Figure 3. Effect of centrifugal force on boer goat sperm quality.
A- Progressive motility, B- Overall motility, C- Membrane integrity, D- Acrosome integrity, E-Viability
and F- Concentration. Data are expressed as mean values (n=8). a,b,cValues in each criteria with different
superscripts are statistically significantly different; p < 0.05

![Câu hỏi ôn thi Giải phẫu sinh lý: Tổng hợp [Mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250714/kimphuong1001/135x160/82081752485139.jpg)
![Câu hỏi ôn tập Ký sinh trùng [chuẩn nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250709/kimphuong1001/135x160/6271752031219.jpg)






![Định lượng Zn (kẽm) máu: [Hướng dẫn chi tiết/ Địa chỉ uy tín/ Phương pháp mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250427/thammduongg/135x160/9251745772534.jpg)


![Giáo trình Vi sinh vật học môi trường Phần 1: [Thêm thông tin chi tiết nếu có để tối ưu SEO]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20251015/khanhchi0906/135x160/45461768548101.jpg)





![Bài giảng Sinh học đại cương: Sinh thái học [mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250812/oursky02/135x160/99371768295754.jpg)



![Đề cương ôn tập cuối kì môn Sinh học tế bào [Năm học mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2026/20260106/hoang52006/135x160/1251767755234.jpg)

![Cẩm Nang An Toàn Sinh Học Phòng Xét Nghiệm (Ấn Bản 4) [Mới Nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20251225/tangtuy08/135x160/61761766722917.jpg)

