Oxidation of phenols by laccase and laccase-mediator systems
Solubility and steric issues
Francesca d’Acunzo, Carlo Galli and Bernardo Masci
Dipartimento di Chimica and Centro CNR Meccanismi di Reazione, Universita
`La Sapienza, 00185 Roma, Italy
To investigate how solubility and steric issues affect the
laccase-catalysed oxidation of phenols, a series of oligomeric
polyphenol compounds, having increasing size and
decreasing solubility in water, was incubated with laccase.
The extent of substrate conversion, and the nature of the
products formed in buffered aqueous solutions, were com-
pared to those obtained in the presence of an organic
cosolvent, and also in the presence of two mediating species,
i.e. N-hydroxyphthalimide (HPI) and 2,2,6,6-tetramethyl-
piperidin-1-yloxy (TEMPO). This approach showed not
only an obvious role of solubility, but also a significant role
of the dimension of the substrate upon the enzymatic reac-
tivity. In fact, reactivity decreases as substrate size increases
even when solubility is enhanced by a cosolvent. This effect
may be ascribed to limited accessibility of encumbered sub-
strates to the enzyme active site, and can be compensated
through the use of the appropriate mediator. While TEMPO
was highly efficient at enhancing the reactivity of large, less
soluble substrates, HPI proved less effective. In addition,
whereas the laccase/HPI system afforded the same products
as laccase alone, the use of TEMPO provided a different
product with high specificity. These results offer the first
evidence of the role of oxidation shuttlesthat the media-
tors of laccase may have, but also suggest two promising
routes towards an environmentally friendly process for
kraft pulp bleaching: (a) the identification of mediators
which, once oxidized by laccase, are able to target strategic
functional groups present in lignin, and (b) the introduction
of those strategic functional groups in an appropriate
pretreatment.
Keywords: laccase; phenols; lignin degradation; HPI;
TEMPO.
Lignin is a three-dimensional, insoluble aromatic polymer
that constitutes 15–33% of biomass. Its structure encom-
passes a number of different types of links between its
constituents, namely ether and C-C diaryl linkages [1].
White-rot fungi achieve the oxidative depolymerization of
lignin by secreting several enzymes, such as lignin peroxidase
[2], manganese peroxidase [3], and laccase (EC 1.10.3.2) [4].
In contrast with lignin peroxidase and manganese peroxi-
dase, laccase can only oxidize the phenolic constituents of
lignin, due to its lower oxidation potential. On the other
hand, it is more readily available and easier to manipulate
than the other two enzymes, and its substrate specificity is
low, as long as a good match of oxidation potentials is
provided [5–8]. In addition, the use of appropriate low
molecular-mass compounds (viz., mediators), in combina-
tion with laccase, makes this enzyme competent for the
oxidation of non-naturalnonphenolic substrates [9–12]. In
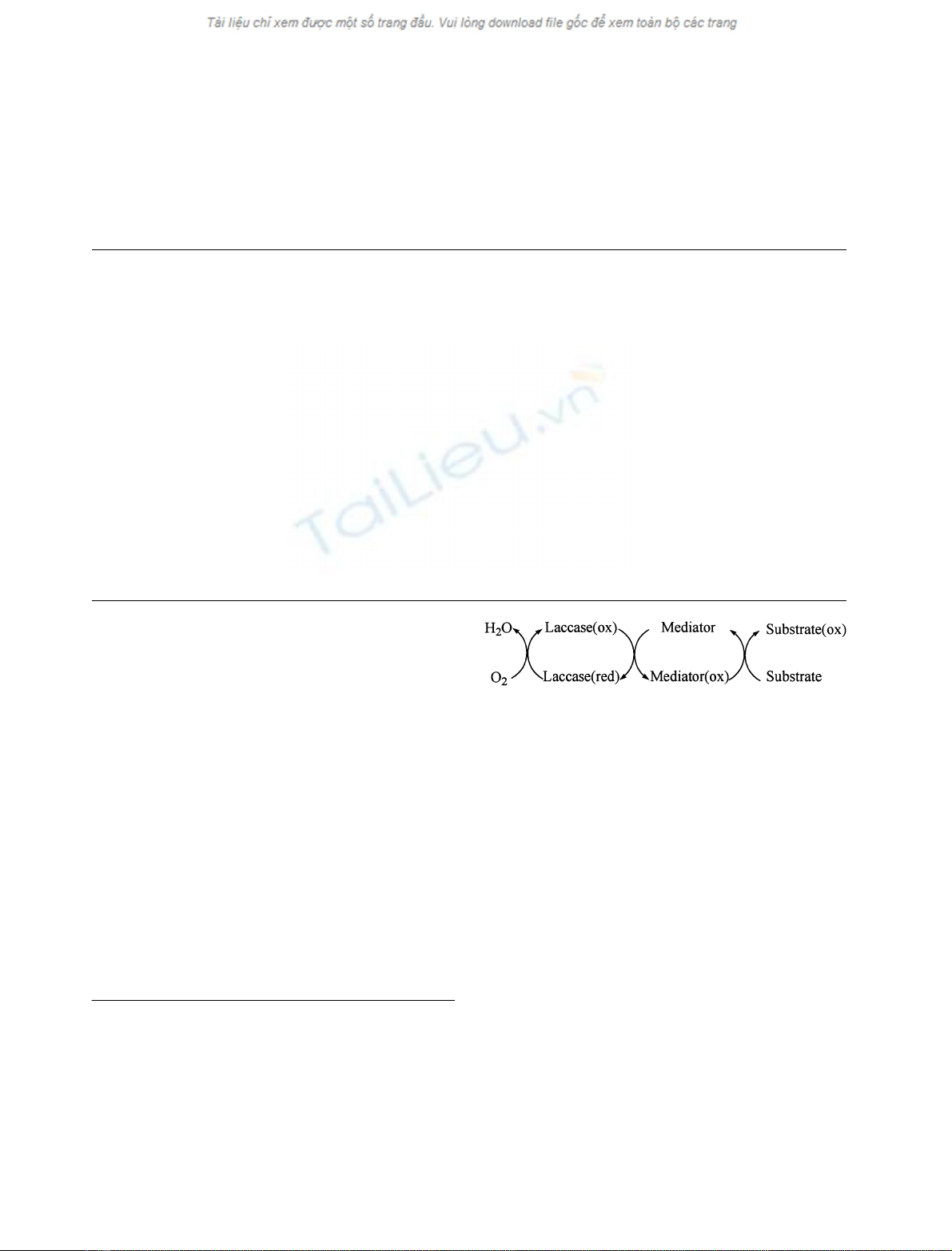
fact, the oxidized mediator (Fig. 1) can rely on an oxidation
mechanism that is not available to the enzyme [13].
Laccase can therefore be turned into a much more
versatile enzyme, and this opens up various possible
applications, as in the textile dye bleaching [14], or for
environmentally respectful kraft pulp delignification [10,15],
or also in selective organic transformations [16–19]. The
study presented here is part of our efforts to elucidate the
mechanisms of action of the laccase/mediator systems [20] in
the oxidation of lignin model compounds, as well as non-
lignin-related structures (Fig. 1).
A conceivable role of the mediator could be that of a sort
of electron shuttlebetween the enzyme and the substrate
[21]. Once the mediator is oxidized by the enzyme, it diffuses
away from the enzymatic pocket and in turn oxidizes
substrates that, due to their size, could not directly enter the
enzymatic pocket. Within this framework, we wished to
investigate the influence of substrate size and solubility on
the effectiveness of laccase oxidation, and also the effect of
mediators endowed with possibly different mechanisms of
action. To this aim, we needed to start from a simple
phenolic structure, which laccase could recognize as a
naturalsubstrate, and modify it into bigger and more
insoluble derivatives. The oligomeric series shown in Fig. 2
served our purposes for the following reasons: (a) each
repeat unit is a phenol, and therefore subject to oxidation
by laccase, at least in terms of redox potential; (b) the
number of repeat units in each oligomer, and therefore its
size, is exactly determined, because directed synthesis and
Fig. 1. Catalytic cycle of a laccase-mediator oxidation system.
Correspondence to C. Galli, Dipartimento di Chimica and Centro
CNR Meccanismi di Reazione, Universita
`La Sapienza,
00185 Roma, Italy. Fax: + 39 06 490421,
E-mail: carlo.galli@uniroma1.it
Abbreviations:HPI,N-hydroxyphthalimide; TEMPO, 2,2,6,6-
tetramethylpiperidin-1-yloxy; ABTS, 2,2¢-azinobis-(3-ethylbenzothi-
azoline-6-sulfonate).
(Received 18 June 2002, revised 9 September 2002,
accepted 12 September 2002)
Eur. J. Biochem. 269, 5330–5335 (2002) FEBS 2002 doi:10.1046/j.1432-1033.2002.03256.x
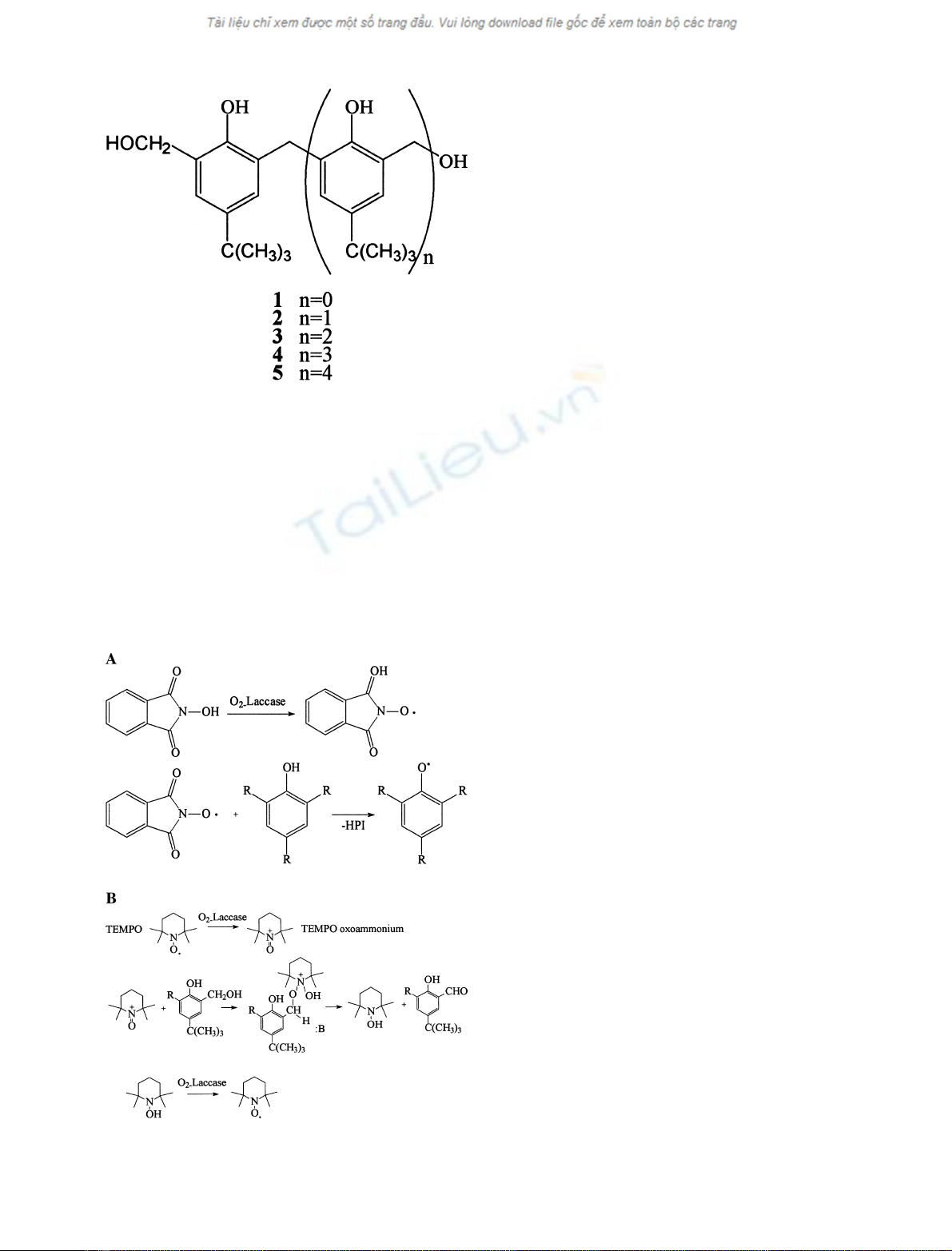
suitable purification allowed pure monodisperse oligomers
to be available; (c) solubility in water decreases as size
increases; and (d) o-o-p-substitution should inhibit C-C
diaryl bond formation, a well-known reaction pathway of
phenoxy radicals [1,12].
We chose N-hydroxyphtalimide (HPI) and 2,2,6,6-
tetramethylpiperidin-1-yloxy free radical (TEMPO) as
mediators, since they greatly differ both in their mechan-
ism of action and in their specificity. In fact, HPI is
representative of a mediator that, after having been oxidized
by laccase, should only induce the generation of phenoxy
radical(s) from the substrate (Fig. 3A) [18,20,22–24]. This
radical role of the oxidized HPI would be comparable to the
naturaloxidation role of the enzyme, with the only
difference of presenting fewer stringent steric and solubility
requirements. The phenoxy radical of the product, in turn,
should evolve towards end-products with little or no further
intervention from HPI.
On the other hand, TEMPO is representative of a
mediator that, by selectively interacting with specific func-
tional groups [25] (i.e. alcohols) (Fig. 3B), not only acts as a
shuttle for the oxidizing power of laccase towards insoluble
or bulky substrates, but also induces the formation of
different products than the physiologicalones. This aspect
may prove useful for synthetic purposes, as well as in lignin
degradation. The mechanism reported in Fig. 3B matches
the well-established one reported for the oxidation of
alcohols by catalytic amounts of TEMPO with stoichio-
metric co-oxidants [25–27]. In all these cases the oxoam-
monium form of TEMPO is involved. In our particular
case, laccase would be the catalytic oxidant of TEMPO [17].
Following a nucleophilic attack of the lone-pair of the
alcohol onto the TEMPO-oxoammonium ion, the interme-
diate adduct is deprotonated at the a-C-H benzylic bond by
the base-form of the buffer B [17,25–27].
In order to decouple the effect of increasing substrate-size
and decreasing solubility, the oxidations were carried out in
1 : 1 aqueous buffer/dioxane, and the results compared with
those obtained in 100% aqueous buffer.
MATERIALS AND METHODS
Laccase
Laccase from Poliporus pinsitus was kindly donated by
Novo Nordisk Biotech and purified by ion-exchange
chromatography on Q-Sepharose by elution with phosphate
buffer [5,20], and an activity of 10 000 UÆmL
)1
determined
spectrophotometrically by the standard reaction with ABTS
[28]. Laccase having an absorption ratio A
280
/A
610
of 20–30
was considered sufficiently pure [5].
Materials
HPI and TEMPO were used as received from Aldrich. The
solvents were from Aldrich, Merck or Carlo Erba.
Synthesis of substrates
Compounds 2–4 were prepared according to a literature
procedure [29]. The latter procedure was also extended to
the stepwise synthesis of compound 5, the pentaphenol
condensation product obtained from compound 3and
4-tert-butylphenol being bis-hydroxymethylated. The purity
of these compounds (> 96%) was checked by
1
HNMR
and HPLC.
Spectrophotometric determination of laccase activity
The activity of laccase was determined by following the rate
of oxidation of 2,2¢-azinobis-(3-ethylbenzothiazoline-6-
sulfonate) (ABTS) to ABTS radical cation (ABTS
•+
), by
plotting the absorbance at 414 nm against time [28].
The extinction coefficient of ABTS
•+
at 414 nm is
3.15 ·10
)4
M
)1
Æcm
)1
. ABTS (Aldrich) was recrystallized
Fig. 3. Mechanisms of the O
2
-laccase-HPI (A) and O
2-
laccase-
TEMPO (B) oxidation of substrates 1–5 (Fig. 2).
Fig. 2. Oligomeric compounds 1–5.
FEBS 2002 Oxidation of phenols (Eur. J. Biochem. 269) 5331

from ethanol prior to use. A stock solution was prepared by
dissolving 3 mg of ABTS in 10 mL 0.1
M
citrate buffer at
pH 5.0; 200 lL of the stock solution were added to 3 mL
citrate buffer in a quartz cuvette (10 mm pathlength); 1 lL
of laccase solution, approximately 10–20 UÆmL
)1
,was
then added, and the initial rate of ABTS
•+
formation
was determined. The 10–20 UÆmL
)1
laccase activity was
achieved by diluting the purified laccase solution in citrate
buffer.
Evaluation of substrates solubility
The solubility of substrates 2,3,5was evaluated by UV-Vis
spectrometry. A Hewlett Packard 8453 diode-array single
beam spectrophotometer was used. A 2.0-m
M
stock solution
of each substrate was prepared in dioxane; aliquots (5.0 lL)
of the stock solution were added to 2.5 mL of 1 : 1 (v/v) 0.1
M
citrate buffer pH 5.0/dioxane mixed solvent in a 10-mm
quartz cuvette. Spectra were recorded in the 220–320 nm
range, and the absorbance at 280 nm was plotted against
concentration.
Oxidations catalysed by laccase and laccase/mediator
systems
In a typical experiment, 60 lmol of substrates 1–5were
weighedina2-mLscrew-capvialequippedwithastirring
bar. In the experiments with mediators, 20 lmol HPI or
TEMPO were also added. In the experiments with aqueous
solvent only, 0.3 mL of 0.1
M
citrate buffer, pH 5.0, were
added at this point, followed by 9–10 U of purified laccase.
In the experiments with the mixed solvent, 0.15 mL of
dioxane were added first, followed by an equal amount of
citrate buffer and 9–10 U laccase. The reaction mixture was
allowed to react at room temperature for 24 h. The vials
were left uncapped and vigorous stirring was maintained in
order to ensure oxygen saturation.
HPLC determination of substrate conversion
Substrate consumption after a 24-h reaction time was
determined using a Hewlett-Packard 1050 HPLC system
(pump, detector, and solvent delivery system) equipped with
a Supelcosil LC-18-DB 25 cm ·4.6 mm column and a HP
3395B integrator. The analyses were carried out with
gradients of water/methanol/isopropanol mixtures, contain-
ing 0.03% trifluoroacetic acid, at 0.5–1 mLÆmin
)1
flow rate.
Quantitation of unreacted substrate was achieved by using
2-bromonaphtalene (Aldrich) as the internal standard. The
standard was added to the reaction crude, which was then
diluted in the mobile phase and filtered through 0.2 lm
Teflon syringe filters (Superchrom Varisep) prior to
analysis.
Product analysis: liquid mass spectrometry (LC-MS)
The analysis was carried out using a triple quadrupole
Perkin Elmer Sciex API 365 spectrometer with a turbo–ion
spray interface. Samples were diluted in HPLC-grade
methanol and filtered through 0.2 lm Teflon syringe filters
prior to injecting. The samples were directly injected into the
ion spray chamber without chromatographic separation.
Product analysis:
1
H-NMR
Samples were dissolved in dimethylsulfoxide-d
6
(Merck)
and spectra were acquired using a Varian 300 MHz
spectrometer with a Mercury console.
RESULTS AND DISCUSSION
Solubility of substrates
This was assessed by a UV-Vis spectrophotometric experi-
ment, aimed at verifying that the substrates were soluble in
the buffer/dioxane mixed solvent up to the concentration
used in the laccase-catalysed reaction. We checked that the
absorbance at 280 nm, corresponding to the maximum
absorbance of the substrates, increases linearly with sub-
strate concentration without scatter from precipitation.
Furthermore, we checked that the absorbance falls to zero
outside the peak, i.e. that no wavelength-independent
turbidity arises from precipitation. We thus verified that
solutions of substrates 2–5as concentrated as 85 l
M
can be
prepared. However, the solution containing substrate 5
became visibly turbid after 24 h. We therefore concluded
that the heaviest of our substrates can yield over-saturated
solutions in the buffer/dioxane mixed solvent, at the
concentrations used for the oxidation reaction.
Substrate consumption
Table 1 summarizes the results of our laccase oxidations of
substrates 1–5, and the effect of the cosolvent and of the
mediators on the amount of substrate metabolized by the
enzyme.
(a) Laccase and laccase/HPI. In general, the use of the
buffer/dioxane mixed solvent enhances substrate conversion
both with and without mediator. The monomeric substrate
Table 1. Percent of substrate metabolized by laccase or laccase/mediator systems with and without an organic cosolvent. Substrate recovery was
determined by HPLC.
No mediator HPI TEMPO
Substrate Buffer Buffer/dioxane Buffer Buffer/dioxane Buffer Buffer/dioxane
1100 100 – – – –
233 96 – 100 – 96
318 55 20 60 32 95
400 0 0 687
500 0 0 040
5332 F. d’Acunzo et al.(Eur. J. Biochem. 269)FEBS 2002


























