Purification and cDNA cloning of a cellulase from abalone
Haliotis discus hannai
Ken-ichi Suzuki, Takao Ojima and Kiyoyoshi Nishita
Laboratory of Biochemistry and Biotechnology, Graduate School of Fisheries Sciences, Hokkaido University, Japan
A cellulase [endo-b-1,4-
D
-glucanase (EC 3.2.1.4)] was iso-
lated from the hepatopancreas of abalone Haliotis discus
hannai by successive chromatographies on TOYOPEARL
CM-650M, hydroxyapatite and Sephacryl S-200 HR. The
molecular mass of the cellulase was estimated to be
66 000 Da by SDS/PAGE, thus the enzyme was named
HdEG66. The hydrolytic activity of HdEG66 toward carb-
oxymethylcellulose showed optimal temperature and pH at
38 C and 6.3, respectively. cDNAs encoding HdEG66 were
amplified by the polymerase chain reaction from an abalone
hepatopancreas cDNA library with primers synthesized on
the basis of partial amino-acid sequences of HdEG66. By
overlapping the nucleotide sequences of the cDNAs, a
sequence of 1898 bp in total was determined. The coding
region of 1785 bp located at nucleotide position 56–1840
gave an amino-acid sequence of 594 residues including the
initiation methionine. The N-terminal region of 14 residues
in the deduced sequence was regarded as the signal peptide as
it was absent in HdEG66 protein and showed high similarity
to the consensus sequence for signal peptides of eukaryote
secretory proteins. Thus, matured HdEG66 was thought to
consist of 579 residues. The C-terminal region of 453 residues
in HdEG66, i.e. approximately the C–terminal three quar-
ters of the protein, showed 42–44% identity to the catalytic
domains of glycoside hydrolase family 9 (GHF9)-cellulases
from arthropods and Thermomonospora fusca. While the
N-terminal first quarter of HdEG66 showed 27% identity to
the carbohydrate-binding module (CBM) of a Cellulomonas
fimi cellulase, CenA. Thus, the HdEG66 was regarded as the
GHF9-cellulase possessing a family II CBM in the N-ter-
minal region. By genomic PCR using specific primers to the
3¢-terminal coding sequences of HdEG66-cDNA, a DNA of
2186 bp including three introns was amplified. This strongly
suggests that the origin of HdEG66 is not from symbiotic
bacteria but abalone itself.
Keywords: cellulase; abalone; invertebrate; cDNA cloning;
cellulase gene.
Cellulase (endo-b-1,4-
D
-glucanase) is an enzyme which
hydrolyzes internal b-1,4-glycoside linkages of cellulose
chains [1]. The cellulase has been shown to exist not only in
plants [2], molds [3], fungi [1], bacteria [1] and protista [4],
but also in herbivorous invertebrates, such as arthropods
[5–7], nematodes [8] and mollusks [9–14]. Most cellulases
from microorganisms are composed of a catalytic domain
and ancillary domains such as CBMs and linkers, while the
invertebrate cellulases except for two nematode enzymes
have just a catalytic domain [1,8,14]. The origin of the
invertebrate cellulases was initially explained as products of
symbiotic microorganisms in the intestine or contamination
by foods [15,16]. However, those cellulases have become
considered to be the products of invertebrates themselves, as
animals bred in the presence of antibiotic could produce
cellulases [17] and cellulase genes were cloned from termite
[18,19], crayfish [20], nematode [21], and mussel [22].
According to the criteria based on hydrophobic cluster
analysis [23], termite and crayfish cellulases are classified
into the GHF9 subfamily which includes the majority of
cellulases from plants, bacteria, and a slime mold [24].
Nematode cellulases are classified into GHF5 which
includes some bacterial and fungal cellulases [21]. On the
other hand, a thermostable and low molecular mass (20-
kDa) cellulase was recently isolated from blue mussel and
the primary structure was determined [13,22]. Origin of the
mussel cellulase was also investigated by genomic PCR
similar to the case of arthropod cellulases. According to the
primary structure analysis, the mussel cellulase is classified
into the GHF45 subfamily 2, being distinct from the
arthropod ones that are classified into GHF9. This leads us
to consider that molluscan cellulases possess somewhat
different properties and a different evolutionally origin from
arthropod ones. However, at present there is little informa-
tion about the biochemical properties and primary struc-
tures of molluscan cellulases to assess the fundamental
differences between molluscan and other invertebrate cellu-
lases.
Therefore, in the present study, we attempted to isolate a
cellulase from abalone Haliotis discus hannai which is one of
the most common and valuable herbivorous molluscs in
Japan, and determine its primary structure. In addition, we
investigated the existence of a cellulase gene in abalone
chromosomal DNA by genomic PCR.
Correspondence to T. Ojima, Laboratory of Biochemistry and
Biotechnology, Graduate School of Fisheries Sciences,
Hokkaido University, Hakodate, Hokkaido 041-8611, Japan.
Fax: + 81 138 40 8800, Tel.: +81 138 40 8591,
E-mail: ojima@fish.hokudai.ac.jp
Abbreviations: CBM, carbohydrate-binding module; GHF, glycoside
hydrolase family; CMC, carboxymethylcellulose.
Enzymes: endo-b-1,4-
D
-glucanase (EC 3.2.1.4).
(Received 9 October 2002, revised 3 December 2002,
accepted 20 December 2002)
Eur. J. Biochem. 270, 771–778 (2003) FEBS 2003 doi:10.1046/j.1432-1033.2003.03443.x

Materials and methods
Materials
Living abalones were purchased from a local market in
Hakodate, Hokkaido prefecture, Japan. CMC (medium
viscosity) was purchased from ICN Bio medicals, Inc. (OH,
USA), TOYOPEARL CM-650
M
was from Toyo Soda Mfg,
Co. (Tokyo, Japan), Sephacryl S-200 HR was from Amer-
sham Pharmacia Biotech AAB (NJ, USA), and Hydroxy-
apatite (Fast Flow Type) was from Wako pure chemical
industries Ltd. (Osaka, Japan). Lysylendopeptidase, Oligo-
tex-dT (30), TaKaRa Taq DNA polymerase, 5¢-Full RACE
and 3¢-Full RACE kits, and restriction endonucleases were
purchased from TaKaRa (Tokyo, Japan). pCR-TOPO 2.1
TA cloning kit was purchased from Invitrogen (CA, USA).
The other chemicals used were reagent grade from Wako
Pure Chemical industries Ltd. (Osaka, Japan).
Determination of enzymatic activity
Cellulase activity was assayed in a 1-mL of reaction mixture
containing 0.5% CMC, 10 m
M
sodium phosphate (pH 7.0),
and an appropriate amount of enzyme at 30 C. The
reducing sugar liberated by hydrolysis of CMC was
determined by the method of Nelson and Somogyi [25].
One unit of cellulase was defined as the amount of enzyme
that liberates reducing sugars equivalent to 1.0 lmol of
glucose per min under the conditions described above.
Temperature dependence of cellulase activity was assayed at
4–80 C and pH 7.0. Thermal stability of cellulase was
assayed by measuring remaining activity of the enzyme that
had been incubated at 4–70 C for 30 min. pH dependence
of cellulase activity was assayed at 30 C in reaction mixtures
adjusted at pH 3.0–9.0 with 10 m
M
sodium phosphate.
Amino-acid sequencing
The N-terminal amino-acid sequence of intact enzyme was
determined with the sample electrically transferred to a
poly(vinylidene difluoride) membrane after SDS/PAGE
using an ABI 473 A protein sequencer (Applied Biosystems,
CA, USA). For the analysis of internal amino-acid
sequences, the enzyme was digested with 1/100 (w/w) of
lysylendopeptidase at 37 C for 2 h. The fragments were
separated by HPLC (LP-1000, EYLA, Tokyo, Japan)
equipped with Mightysil RP-18 GP column (150 ·4.6 mm)
(KANTO CHEMICAL CO., INC, Tokyo, Japan) and
subjected to the protein sequencer.
SDS/PAGE
SDS/PAGE was performed with 10% polyacrylamide gel
according to the method of Porzio and Pearson [26]. After
the electrophoresis, the gel was stained with 0.1% Coomas-
sie Brilliant Blue R-250 in 50% methanol-10% acetic acid,
and destained with 5% methanol-7% acetic acid.
Zymography
Zymography for cellulase was carried out by the method of
Be
´guin [27] with slight modifications as follows: The enzyme
was run on SDS/PAGE at 4 C and the gel was washed
with 100 mL of 10 m
M
sodium phosphate (pH 7.0))25%
2-propanol by gently shaking at 4 Cfor30mintoremove
SDS. This washing was repeated once more and the gel was
equilibrated with 10 m
M
sodium phosphate (pH 7.0) at
4C for 30 min to accomplish renaturation of the enzyme.
Then, the gel was laid on 2% agar gel (5 mm thick)
containing 0.1% CMC and 10 m
M
Tris/HCl (pH 7.5)
which was solidified in Petri dish (/20 cm). After the
incubation at 37 C for 3 h, the overlaid gel was removed
and the agar replica gel was stained with 0.1% Congo Red
aqueous solution. Location of the enzyme was detected as
unstained bands.
Determination of protein concentration
Protein concentration was determined by the biuret method
[28] or the method of Lowry et al. [29] using bovine serum
albumin fraction V as a standard protein.
cDNA cloning
Construction of the cDNA library and cloning of cellulase
cDNA was achieved as follows: Total RNA was extracted
from 1 g of abalone hepatopancreas by the ganidinium
thiocyanate-phenol method [30] and mRNA was selected
with Oligotex-dT (30) from the total RNA according to the
manufacturer’s protocol. Double-stranded cDNA was syn-
thesized from the mRNA with a cDNA synthesis kit
(TaKaRa, Tokyo, Japan) and used as an abalone cDNA
library. cDNAs encoding abalone cellulase were amplified
by PCR from the cDNA library with degenerated primers
synthesized on the basis of partial amino-acid sequences of
the cellulase. PCR was carried out in a 50-lL of reaction
mixture containing 50 m
M
KCl, 10 m
M
Tris/HCl (pH 8.3),
2m
M
each of dATP, dTTP, dGTP and dCTP, 1.2 m
M
MgCl
2
,2pmolÆmL
)1
primers, 1 ngÆmL
)1
template DNA,
and 0.05 unitsÆmL
)1
TaKaRa Taq DNA polymerase. A
successive reaction at 95 C for 30 s, 45 Cfor60sand
72 C for 90 s was repeated for 30 cycles with a PC 700
Program Incubator (ASTEC, Fukuoka, Japan). cDNAs for
5¢-and 3¢-terminal regions of mRNA were amplified with a
5¢-Full RACE kit and a 3¢-Full RACE kit (TaKaRa,
Tokyo, Japan), respectively. Genomic PCR was performed
with DNA primers specific to 3¢-terminal regions of the
cellulase cDNA and abalone chromosomal DNA prepared
from the adductor muscle by the conventional method [31].
The PCR products were cloned with a pCR-TOPO 2.1 TA
cloning kit (Invitrogen, CA, USA) and sequenced using a
BigDye-terminator Cycle sequencing kit (Applied Biosys-
tems, CA, USA) and an ABI 310 DNA sequencer (Applied
Biosystems, CA, USA).
Results
Purification of cellulase from abalone hepatopancreas
Hepatopancreas (125 g) dissected from 10 abalones (aver-
age shell size: 8 ·6 cm) were cut into small pieces with
scissors and suspended in 250 mL of 10 m
M
sodium
phosphate (pH 7.0) containing 0.2% sodium azide, 1 m
M
phenylmethanesulfonyl fluoride and 1 m
M
EDTA. After
772 K.-i. Suzuki et al.(Eur. J. Biochem. 270)FEBS 2003
the extraction at 4 C for 30 min, the extract was centri-
fuged at 10 000 gfor 15 min. The supernatant was applied
to a TOYOPEARL CM-650M column (2.0 ·15 cm) pre-
equilibrated with 10 m
M
sodium phosphate (pH 7.0), and
proteins adsorbed were eluted with a linear gradient of
0–200 m
M
NaCl in 10 m
M
sodium phosphate (pH 7.0). As
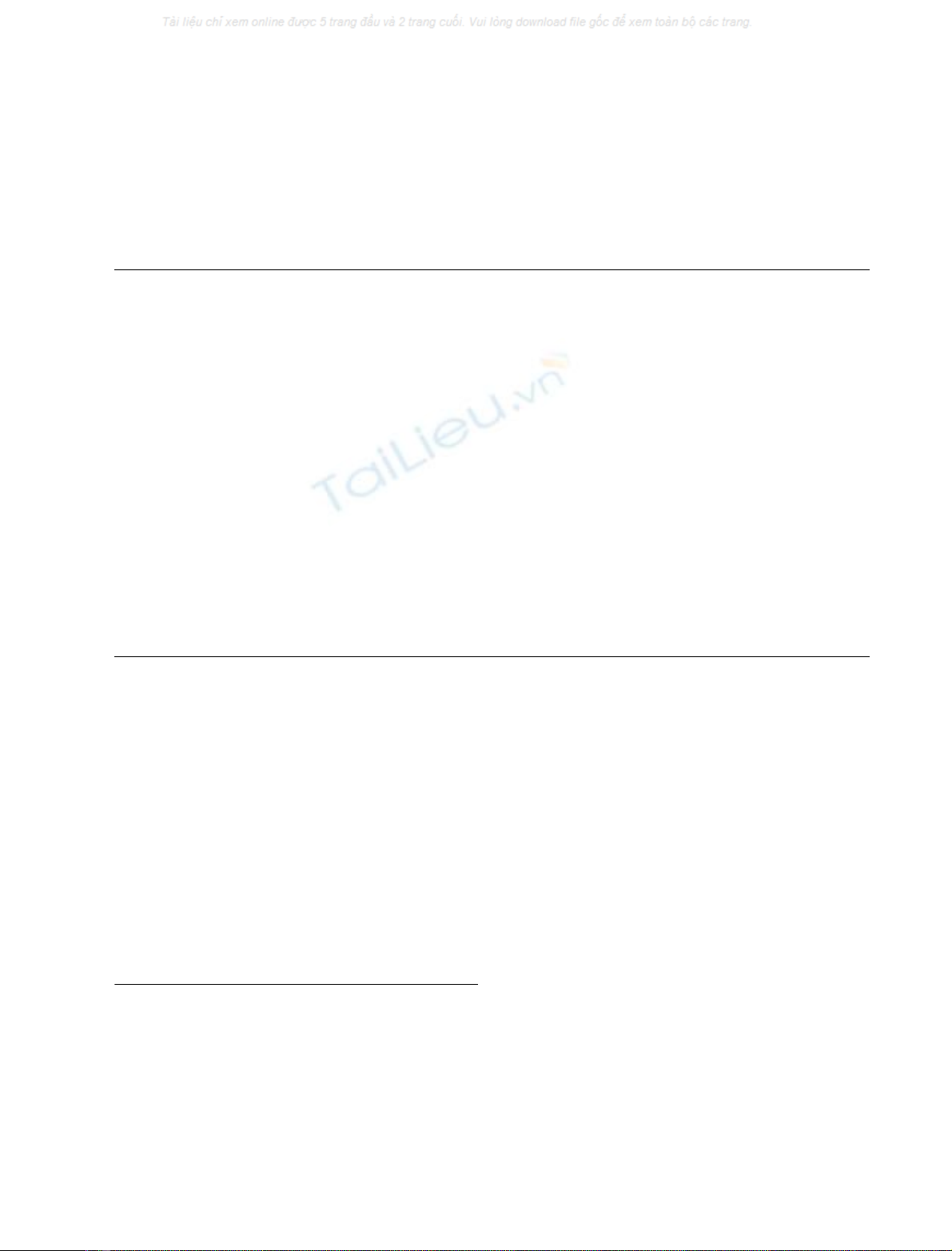
shown in Fig. 1, three fractions showing hydrolytic activity
toward CMC, namely CM-I–III fractions, were eluted.
According to SDS/PAGE followed by zymography, the
CM-I and -II were found to contain 66 000, 75 000, and
100 000 Da proteins with cellulase activity in substantial
amounts. However, the CM-III fraction contained only the
66 000 Da protein in fairly high purity. Therefore, in the
present study, we focused on the 66 000-Da cellulase
(named HdEG66) in the CM-III fraction and attempted
to isolate it.
The CM-III fraction, i.e. fractions 19–24, was applied to a
hydroxyapatite column (1.5 ·20 cm) pre-equilibrated with
10 m
M
potassium phosphate (pH 7.0), and adsorbed pro-
teins were eluted with a linear gradient of 10–300 m
M
potassium phosphate (pH 7.0). As shown in Fig. 2, the
HdEG66 was eluted as a major peak at around 0.18
M
potassium phosphate, i.e. fractions 25–29. As these fractions
were still contaminated by the small amount of 25 000 Da
protein, they were lyophilized and subjected to gel-filtration
through Sephacryl S-200 HR. Consequently, the HdEG66
was eluted in a single major peak and showed a single band
of 66 000 Da in both the SDS/PAGE and zymogram
(Fig. 3). According to the eluting position in the gel-
filtration, molecular mass of the HdEG66 was estimated to
be 66 000 Da which was fairly consistent with that estima-
ted by SDS/PAGE. This indicates that HdEG66 is a
monomeric enzyme. The yield and purity of the HdEG66 in
respective purification steps are summarized in Table 1. The
purified HdEG66 showed a specific activity of 13.9 UÆmg
)1
,
which is approximately 95-fold higher than that of crude
extract. The optimal temperature and pH of the HdEG66
were at 38 C and pH 6.3, respectively, and the enzyme was
stable to heating at 30 C for 30 min (data not shown).
Partial amino-acid sequence of HdEG66
Partial amino-acid sequences of the HdEG66 were analyzed
in order to design PCR primers for the amplification of
HdEG66-cDNA. To analyze the N-terminal sequence, the
HdEG66 was blotted onto a poly(vinylidene difluoride)
membrane and subjected to the protein sequencer. Conse-
quently, an amino-acid sequence of 14 residues was
identified as VDVTISNHWDGGFQ (Table 2). Then, in
order to analyze the internal amino-acid sequences, the
HdEG66 was digested with lysylendopeptidase and the
Fig. 1. TOYOPEARL CM-650M column chromatography of abalone
crude extract. Crude extract from abalone hepatopancreas was applied
to a TOYOPEARL CM-650M column (2.0 ·15 cm) and eluted with
a 0–0.2
M
NaCl linear gradient in 10 m
M
sodium phosphate (pH 7.0)
at a flow rate of 30 mLÆh
)1
. Each fraction contains 5.0 mL. The SDS
gel electrophoretic patterns of the sample before chromatography (Cr)
and fractions indicated by the arrows a–j are shown in the inset.
M, molecular mass markers; 15 K ¼15 000 Da etc.
Fig. 2. Purification of abalone cellulase by hydroxyapatite column
chromatography. The CM-III fraction in TOYOPEARL CM-650M
chromatography was applied to a hydroxyapatite column
(1.5 ·20 cm) and eluted with a 0.01–0.3
M
potassium phosphate
(pH7.0)ataflowrateof30mLÆh
)1
. Each fraction contains 5.0 mL.
The SDS gel electrophoretic patterns of fractions indicated by the
arrowsa–iareshownintheinset.Thefractionsc–ewerepooled.
Fig. 3. Purification of abalone cellulase by Sephacryl S-200 HR gel
filtration. The cellulase fraction obtained in the hydroxyapatite column
chromatography was concentrated by lyophilization and then applied
to a Sephacryl S-200 HR column (2.0 ·140 cm). Each fraction con-
tains 5.0 mL. V
0
is the void volume of the column. The SDS gel
electrophoretic and zymographic patterns of fractions indicated by the
arrows a–c are shown in the inset. The fractions a–c were pooled as the
purified HdEG66.
FEBS 2003 cDNA cloning of abalone cellulase (Eur. J. Biochem. 270) 773

fragments were isolated by HPLC. Among the fragments,
LP1-LP9 fractions were subjected to sequencing (Table 2).
According to database searches on DDBJ, GenBank and
EMBL, these sequences were found to show 42–80%
identity to the amino-acid sequences of termite and
cockroach cellulases. Among the fragments, LP5 and LP6
were considered to be derived from middle and C-terminal
region of the HdEG66, respectively, from the sequence
similarity to the termite cellulase. Then, a forward primer F1
was synthesized on the basis of the N-terminal sequence of
the HdEG66, while reverse primers R1 and R2 were
synthesized on the basis of sequences of LP5 and LP6,
respectively (Table 2).
PCR amplification of HdEG66-cDNA
cDNAs encoding the N-terminal region of the HdEG66
were amplified by PCR using the F1–R1 primer pair. This
PCR gave a cDNA with approximately 1000 bp named
Hd1-DNA. The Hd1-DNA was cloned with a TOPO
cloning kit and sequenced (Fig. 4A). The amino-acid
sequence deduced from the Hd1-DNA corresponded to
the N-terminal 335 amino-acid sequence of the HdEG66.
Next, in order to obtain cDNAs encoding C-terminal region
of the HdEG66, a forward specific primer F2 was newly
synthesized and PCR was performed using the F2-R2
primer pair. Thus, Hd2-DNA of 477 bp encoding the
C-terminal 159 amino acids of the HdEG66 was amplified.
Finally, 5¢-and3¢-RACE PCRs were performed using
primers shown in Table 2, and Hd5RACE-DNA and
Hd3RACE-DNA for 5¢-and3¢-terminal regions were
amplified, respectively. By combining the nucleotide
sequences of the Hd5RACE-DNA, Hd1-DNA, Hd2-
DNA and the Hd3RACE-DNA in this order, the nucleo-
tide sequence of total 1898 bp was determined (Fig. 5). The
reliability of the nucleotide sequence was confirmed with
HdFull-DNA which was amplified with the specific primer
pair, FLF1–FLR1 (Table 2 and Figs 4 and 5). The
translational initiation codon ATG was found in nucleotide
positions from 56 to 58 and termination codon TAA from
1838 to 1840 (Fig. 5). In the 3¢-terminal region, a putative
polyadenylation signal sequence AATAAA and a
poly(A+) tail were found. These structural characteristics
indicate that the HdEG66 cDNA is not derived from
prokaryote like intestinal bacteria. The translational region
of 1785 bp gave an amino-acid sequence of 594 residues. All
the amino-acid sequences determined with lysylendopepti-
dase fragments, LP1–LP9, are found in the deduced
sequence, indicating that the thus cloned cDNAs are of
the HdEG66 protein. It is noteworthy that the N-terminal
15 residues in the deduced sequence are absent in the
HdEG66 protein. According to the sequence comparison
with the consensus sequence for signal peptides of eukaryote
secretory proteins [32], the N-terminal region of 14 residues
except for the initiation methionine is regarded as the signal
peptide of the HdEG66. Further, the inconsistent residue
Table 2. Partial amino acid sequences of HdEG66 and nucleotide sequences of primers. W¼A/T, Y ¼C/T, H ¼A/C/T, H ¼A/C/T, R ¼A/G,
S¼C/G and N ¼A/G/C/T.
Peptides Sequences Primer names DNA sequences
Intact VDVTISNHWDGGFQ F1 ACNATHWSNAAYCAYTGGGA
LP1 DAYATTK
LP2 WRGDSALGDK
LP3 GDNGEDLTGGWY
LP4 TEVEGFFK
LP5 YPGIYSSSIQDAGQFYSSSGYK R1 RTARAAYTGNCCNGCRTCYTG
LP6 WAVEQMNYILGDNK R2 CATYTGYTCNACNGCCCAYTT
LP7 AWAWALGWDDK
LP8 GYHENA
LP9 WPLDYFL
F2 GCCACACTTCTGTCAACATCC
3RAC TTCTTCAAGGGCTGGCTCCCT
3AP CTGATCTAGAGGTACCGGATCC
5RACF ATCCTCACGAACAAGCAG
5RACR GATCGCGATGCAGGCCTT
FLF1 GGACGACTACAGCGTCTTCAGTAGA
FLR1 TCCAAACAGTCAGTTTCTTAACCGT
Table 1. Purification of cellulase from abalone Haliotis discus hannai.Oneunitofcellulasewasdefinedastheamountofenzymethatliberates
reducing sugars equivalent to 1.0 lmol of glucose per min.
Purification step
Total protein
(mg)
Specific activity
(unitsÆmg
)1
)
Total activity
(units)
Purification
(fold)
Yield
(%)
Crude extract 1470 0.15 220 1 100
CM-III fraction 13.4 2.95 40 20 18
Hydroxyapatite 4.14 4.00 16 27 7
Sephacryl S-200 1.06 13.9 15 93 7
774 K.-i. Suzuki et al.(Eur. J. Biochem. 270)FEBS 2003
between the deduced sequence and the sequence of LP7
peptide was found at the amino-acid position 388. Namely,
the neighboring residue of LP7 toward the N-terminus
should be lysine because LP7 is a fragment produced by
lysylendopeptidase digestion. However, the corresponding
residue is not lysine but asparagine in both the deduced
sequence and the amino-acid sequence of LP8. We now
consider that this inconsistency has arisen from hetero-
geneity of the HdEG66, e.g. coexistence of proteins with
lysine and asparagine at the position 338 in the HdEG66
preparation.
Amplification of HdEG66 gene from abalone
chromosomal DNA
The existence of HdEG66 gene in the abalone chromosomal
DNA was examined by genomic PCR using primers, 3RAC
and FLR1, which are specific to the 3¢-terminal region of the
HdEG66-cDNA (Table 2). By PCR, a DNA of 2186 bp
named Hdcel-1 DNA was amplified from the abalone
chromosomal DNA that was prepared from the adductor
muscle. By comparison with the sequence of HdEG66-
cDNA, the Hdcel-1 DNA was revealed to consist of three
introns (each 664 bp, 757 bp, and 229 bp) and 4 exons (each
91 bp, 172 bp, 191 bp, and 82 bp) (Fig. 4B). The positions
of introns in the cDNA sequence are also shown in Fig. 5.
The GU-AG rule in eukaryotic genes is applicable to these
intron-exon junctions. These results indicate that the Hdcel-1
DNA is part of a structural gene for HdEG66 and that
HdEG66 is the product of abalone itself, and is not derived
from symbiotic microorganisms, e.g. intestinal bacteria.
Discussion
In the present study, we have successfully isolated a cellulase
HdEG66 from abalone hepatopancreas. The molecular
mass of HdEG66 was estimated to be 66 000 Da by both
SDS/PAGE and gel-filtration through Sephacryl S-200 HR,
and the optimal temperature and pH were shown to be
38 C and pH 6.3, respectively. In addition, the HdEG66
showed weak hydrolytic activity toward crystalline cellulose
like termite cellulases (data not shown). The HdEG66
showed somewhat larger molecular size compared to the
other invertebrate cellulases, however, the basic properties
of the HdEG66 were fairly similar to those of other
invertebrate cellulases [6,11,12,33].
With cDNAs amplified by PCR, an amino-acid sequence
of 594 residues for the HdEG66 was determined. The
N-terminal region of 15 residues including initiation methi-
onine was absent in the purified HdEG66 protein and
showed the characteristic feature for signal peptides of
eukaryotic secretory proteins [32]. Therefore, this region was
regarded as the signal peptide that was cut away upon
secretion of the HdEG66. Accordingly, the matured
HdEG66 was considered to consist of 579 residues with
the calculated molecular mass of 63 196.88 Da.
By sequence comparison with other invertebrate and
bacterial cellulases, the C-terminal region of 453 residues in
the HdEG66 was regarded as the GHF9-type catalytic
domain i.e. it showed 44, 43, and 42% identity with the
corresponding regions of termite [18], crayfish [20], and
Thermomonospora fusca [34] cellulases, respectively (Fig. 6).
Further, the catalytically important residues in GHF9
cellulases [35–38], i.e. His506, Asp200, Asp203, Asp550 and
Glu559 in the HdEG66 sequence were all conserved
(Fig. 6). Based on these results, we conclude that the
HdEG66 is classified into GHF9. On the other hand,
HdEG66 was found to possess an extended N-terminal
region of 126 residues which is deficient in other invertebrate
cellulases (Fig. 6). This extended region showed sequence
identity of 27% with the CBM attached by a linker in
Cellulomonas fimi CenA [39]. The CBM of CenA belongs
to CBM family II, which is currently the largest among five
principal families, i.e. families I–IV and VI [1]. The family II
CBMs possess strictly conserved four tryptophans and
highly conserved two cysteines that form a disulfide bridge.
In case of N-terminal extended region of the HdEG66, three
out of the four tryptophans are conserved at residues 24, 43,
and 79, although the remaining one is substituted by
aspartic acid at residue 57. While the two cycteines are not
conserved in HdEG66, three cysteines are however present
at residues 33, 58, and 90. In addition, a putative linker
region rich in threonine and glycine residues locates in
the position connecting the N-terminal extended region
and the catalytic domain (Fig. 6). These sequence charac-
teristics strongly suggest that the N-terminal extended
region of the HdEG66 corresponds to a family II CBM
followed by a linker. Accordingly, HdEG66 is considered
to be the first animal cellulase possessing the family II CBM
in the N-terminus of the GHF9-type catalytic domain.
Cellulose-binding ability and other biochemical functions
Fig. 4. Structures of cDNA and genomic fragment for HdEG66. (A)
structure of HdEG66 cDNA. Open and closed boxes indicate trans-
lational and untranslational regions, respectively. Relative positions of
Hd1-DNA, Hd2-DNA, Hd5RACE-DNA, Hd3RACE-DNA, and
HdFull-DNA are indicated as solid lines. Bold lines in both sides of the
cDNAsindicateprimersusedforthePCR.LengthsofthecDNAsare
shown in the parentheses. (B) Structure of the genomic fragment,
Hdcel-1 DNA. Open and closed boxes represent exons and introns,
respectively. The sequence data for HdEG66 cDNA and Hdcel-1
DNA are available from DNA Data Bank of Japan with accession
numbers, AB092978 and AB092979, respectively.
FEBS 2003 cDNA cloning of abalone cellulase (Eur. J. Biochem. 270) 775


























