Purification and characterization of NTPDase1 (ecto-apyrase)
and NTPDase2 (ecto-ATPase) from porcine brain cortex synaptosomes
Filip Kukulski and Michal Komoszyn
´ski
Department of Biochemistry, Institute of General and Molecular Biology, N. Copernicus University, Torun
´, Poland
We purified to homogeneity and characterized NTPDase1
and NTPDase2 from porcine brain cortex synaptosomes.
SDS/PAGE and immunoblotting with antibodies specific
to these enzymes revealed a molecular mass estimated at
72 kDa for NTPDase1 and 66 for NTPDase2. Both
enzymes exhibited kinetic properties typical for all members
of the NTPDase family, e.g. low substrate specificity for tri-
and diphosphonucleosides, divalent cations dependency and
insensitivity towards ATPase inhibitors. The calculated
K
m
value for NTPDase1 in respect to ATP as a substrate
(97 l
M
) was three times lower in comparison to analogous
values for NTPDase2 (270 l
M
). Additionally, NTPDase1
had a three times higher K
cat
/K
m
coefficient than NTPDase2
(860 and 833 lmol productÆs
)1
, respectively). We have also
demonstrated that in spite of differences in the affinity of
ATP for both hydrolases, these enzymes have similar
molecular activity. Taken together, these results indicate that
NTPDase1 would terminate P2 receptor-mediated signal
transmission whereas activity of NTPDase2 may contribute
to decreasing high (toxic) concentrations of ATP and/or to
production of another signal molecule, ADP.
Keywords: central nervous system; extracellular purines; P2
receptors; signal transmission; ecto-nucleoside triphosphate
disphosphohydrolase.
Extracellular ATP and ADP, as well as UTP and UDP
participate in biological signaling (particularly, neurotrans-
mission processes in the central nervous system, CNS) by
activating nucleotide P2 receptors [1,2]. Nucleotide medi-
ated signal transmission is terminated by hydrolysis of
pyrophosphate bonds present in the agonist structure [3,4].
In the CNS, extracellular tri- and diphosphonucleosides are
degraded by three representatives of NTPDase family of
enzymes (NTPDase1–3) [5–7]. NTPDases cloned from CNS
cells are integral cell membrane proteins that share high
amino acid sequence homology [5,7,8]. Multiple sequence
alignments of these enzymes show several regions of amino
acid identity, termed apyrase conserved regions (ACR)
[9–11]. ACR domains are thought to play a critical role in
the binding and hydrolysis of substrates as site directed
mutagenesis within these domains lead to the loss of
biological activity of NTPDases or changed their affinity
towards ATP and ADP [11–15].
NTPDase1 differs from NTPDase2 in respect to reaction
products of ATP hydrolysis and in the ratio of the rate of
ATP hydrolysis to the rate of ADP hydrolysis [16–19].
NTPDase1 degrades ATP and ADP directly to AMP,
whereas NTPDase2 hydrolyses ATP to ADP [17].
NTPDase3 is a functional intermediate between NTPDase1
and 2, characterized by the ATP/ADP ratio of 3 [6].
Coexpression of NTPDase1 and NTPDase2 has been
observed in some nerve structures [5,20]. Hitherto obtained
results strongly indicate that NTPDase1 participates in the
termination of P2 receptor-mediated signal transmission
[4,21,22], whereas the function of NTPDase2 remains a
matter of speculation.
In this work we purified two NTPDases from porcine
brain cortex synaptosomes. The physicochemical and
biochemical properties of homogeneous preparations of
these enzymes allowed us to classify them as NTPDase1 and
2. These results may contribute to the determination of
biological function fulfilled by both ecto-nucleotidases.
Materials and methods
Materials
Analytical grade reagents purchased from Fluka, Serva,
Sigma, Merck, ICN, POCH (Gliwice, Poland) were used.
Pig brains were obtained directly from the slaughterhouse.
Electrophoresis, Western blotting and isoelectric focusing
were performed in a Mini-Protean II apparatus obtained
from Bio-Rad. Qualitative and quantitative purine analysis
was performed using HPLC equipment from Pharmacia
LKB, UV/VIS detector from Shimadzu, Supelcosil
TM
LC-18-DB column purchased from Supelco (15 cm ·
4.6 mm, 5 lm) and computer software
CHROMA
from
PolLab (Warsaw, Poland). MonoQ HR 5/5 column was
obtained from Pharmacia. Nitrocellulose membrane NC2
was obtained from Serva. Silver stain kit and BCIP/NBT
fast tablets (blue tetrazolium and 5-bromo-4-chloro-3-
indolyl phosphate) were provided by Sigma. Ringo
antibodies and BGO were obtained from A. Beaudoin
Correspondence to F. Kukulski, Le Centre Hospitalier Universitaire
de Que
´bec (CHUQ), Centre de recherche
´du pavillon CHUL,
2705 boulevard Laurier, local T1-49, Que
´bec, Canada, G1V 4G2.
Fax: + 1 418 654 2765, Tel.: + 1 418 654 2772,
E-mail: Filip.kukulski@crchul.ulaval.ca
Abbreviations: CNS, central nervous system; BGO, 1-hydroxy-
naphtalene-3,6-disulfonic acid; NEM, N-ethylmaleimide;
TBA, tetrabutyloammonium hydrogen sulfate.
(Received 20 March 2003, revised 29 May 2003,
accepted 30 June 2003)
Eur. J. Biochem. 270, 3447–3454 (2003) FEBS 2003 doi:10.1046/j.1432-1033.2003.03734.x

(Departement de Biologie, Faculte des Sciences, Universite
de Sherbrooke, Quebec, Canada).
Isolation of NTPDases from synaptosomes
Synaptosomes were isolated and purified by the method
of Jones and Matus [23]. NTPDases were extracted from
membranes using 0.9% polydocanol (w/v) in 10 m
M
Hepes/
OH buffer pH 7.6 containing 10% glycerol (v/v), 40 m
M
KCl, 1 m
M
EDTA and 1 m
M
phenylmethanesulfonyl
fluoride. During extraction, a constant ratio of protein/
detergent was maintained at 1 : 3 (w/w). Synaptosomes
were incubated in a detergent solution for 30 min at 0 C,
and subsequently centrifuged for 60 min at 100 000 g
(Beckman centrifuge, 45 Ti rotor). The resulting super-
natant was used for further purification.
NTPDase purification
The solubilized proteins was applied to a column
(5 mm ·1.5 cm) filled with Con A/Sepharose 4B and
equilibrated with 20 m
M
start buffer Tris/HCl pH 7.6
containing 75 m
M
NaCl, 1 m
M
CaCl
2
,1m
M
MgCl
2
,10%
glycerol (v/v) and 0.05% polydocanol (w/v). The column
was washed with start buffer and then glycoproteins were
eluted from the column with 500 m
M
a-methylglucoside in
the start buffer. The NTPDase enriched fractions were
concentrated and separated on a Toyopearl HW55-S gel
(2.5 cm ·60 cm column), equilibrated with 50 m
M
Tris/
maleate buffer pH 7.6 containing 100 m
M
KCl and 0.05%
polydocanol (w/v). The fractions containing ATP/ADPase
activity were concentrated, dialyzed and separated on a
Mono Q HR 5/5 column equilibrated with 20 m
M
Tris/HCl
buffer pH 8.0 with 0.05% polydocanol (w/v). The proteins
were eluted from the column with a following KCl gradient
in the start buffer: 0–10 min 0% KCl, 11–70 min from 0 to
100% (1.5
M
) KCl, 71–80 min from 100 to 0% KCl. All
above described purification procedures were performed at
the flow-rate of 1 mLÆmin
)1
.
Electrophoresis
Electrophoresis under nondenaturing and denaturing con-
ditions was performed according to the procedure described
by Ogita and Markert [24]. The proteins were silver stained
using Sigma Silver Stain Kit according to manufacturer’s
instructions.
Immunoblotting
Proteins separated on a 10% acrylamide SDS gel were
transferred to a Serva nitrocellulose membrane NC2.
Electrotransfer was performed for 14 h at 4 C according
to a procedure described by Towbin et al.[25].The
nitrocellulose membrane after washing and blocking with
skimmed milk was incubated for 4 h in a solution of the
primary rabbit antibody Ringo (anti-ACR4), diluted 2000
times in Tris-buffered saline. After several washes, the
membrane was incubated with alkaline phosphatase conju-
gated goat anti-rabbit Igs at a dilution of 1 : 30 000. The
bands were visualized using blue tetrazolium and 5-bromo-
4-chloro-3-indolyl phosphate (BCIP/NBT Sigma fast
tablets) as the substrates, in accordance to supplier’s
instructions.
Isoelectric focusing of NTPDase1 and NTPDase2 under
nondenaturing conditions
Native isoelectric focusing was performed according to
the method described by Bollag and Edelstein [26]. The
separated proteins were extracted from the gel with a
solution of 0.1% polydocanol (w/v) in 200 m
M
Hepes/OH
buffer pH 7.6. NTPDase activity in extracts was determined
using HPLC.
NTPDase1 and NTPDase2 activity assay
Aliquots of 100 lL mixture containing 50 m
M
Hepes/OH
pH 7.6, 3 m
M
CaCl
2
or 8 m
M
EGTA, 2 m
M
ouabain and
2m
M
nucleotides was preincubated for 10 min at 37 C.
The reaction was initiated by adding 100 lLofenzyme
preparation. Reaction was performed for 30 min. Then the
orthophosphate, nucleotide and nucleoside formed during
reaction were analysed.
Determination of orthophosphate liberated
in the reaction
The reaction was stopped by addition of 100 lL10%SDS
(w/v) with 10 m
M
EDTA to the sample. The liberated
orthophosphate was quantitated according to Hegyvary’s
method [27] modified by Komoszynski and Skalska [28].
The activity of the enzymes was calculated based on a
difference of colour intensity developed for samples con-
taining Ca
2+
or EGTA.
Determination of nucleotide reaction products using
HPLC
Enzymatic reactions were performed as described above
and inhibited by addition of 100 lL incubation mixture to
100 lL cold (0–4 C) 1
M
perchloric acid. The sample was
cooled immediately to 0 C and centrifuged for 2 min in a
microcentrifuge. The supernatant was neutralized with 1
M
KOH (4 C), centrifuged and then lipids were removed by
extraction with n-heptane (5 : 1, v/v). Samples prepared in
such a manner were analysed isocratically in 100 m
M
KH
2
PO
4
/K
2
HPO
4
buffer pH 7.0, containing 5 m
M
EDTA,
2.5% methanol (v/v) and 12.5 m
M
TBA. The flow-rate of
eluent was 1 mLÆmin
)1
. The separated fractions were
analysed spectrophotometrically at k¼260 nm.
Protein determination
Protein was determined by the Bradford method [29] or
with bis-cinchonic acid [30] using bovine serum albumin as
a standard.
Results
Isolation and purification
Polydocanol at a concentration of 0.9% released 70%
of ATP/ADPase active protein from purified synaptic
3448 F. Kukulski and M. Komoszyn
´ski (Eur. J. Biochem. 270)FEBS 2003

membranes. Further purification was performed by affinity,
size-exclusion and ion exchange chromatography. The
specific activity of purified NTPDase1 corresponded to 69
and 49 lmol P
i
Æmin
)1
Æmg
)1
for ATP and ADP as substrates,
respectively. NTPDase2 had specific activity of 80 lmol
P
i
Æmin
)1
Æmg
)1
protein in respect to ATP and 6.7 lmol
P
i
Æmin
)1
Æmg
)1
protein in respect to ADP (Table.1). Both
enzymes were homogeneous in a 5% acrylamide gel under
nondenaturing conditions and in a 10% acrylamide SDS gel
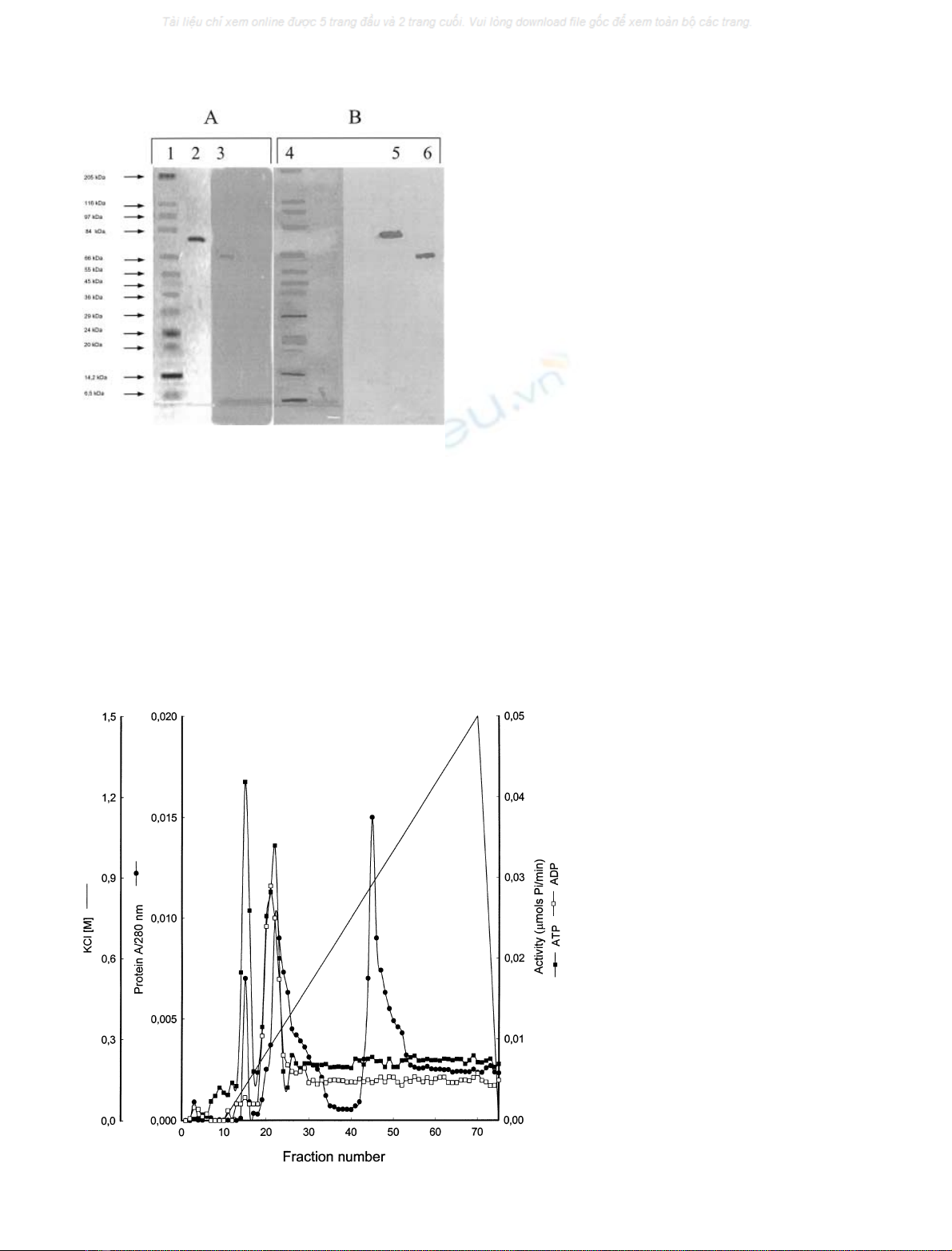
(Figs 1,2A). Ion exchange chromatography on a MonoQ
HR 5/5 column was a crucial step of purification and
separation of synaptosomal NTPDases. Chromatography
on this carrier allowed not only a marked purification of
both proteins but also separation of NTPDase1 from
NTPDase2. The NTPDase2 was eluted from the MonoQ
column with 105 m
M
KCl (Fig. 3). It readily hydrolysed
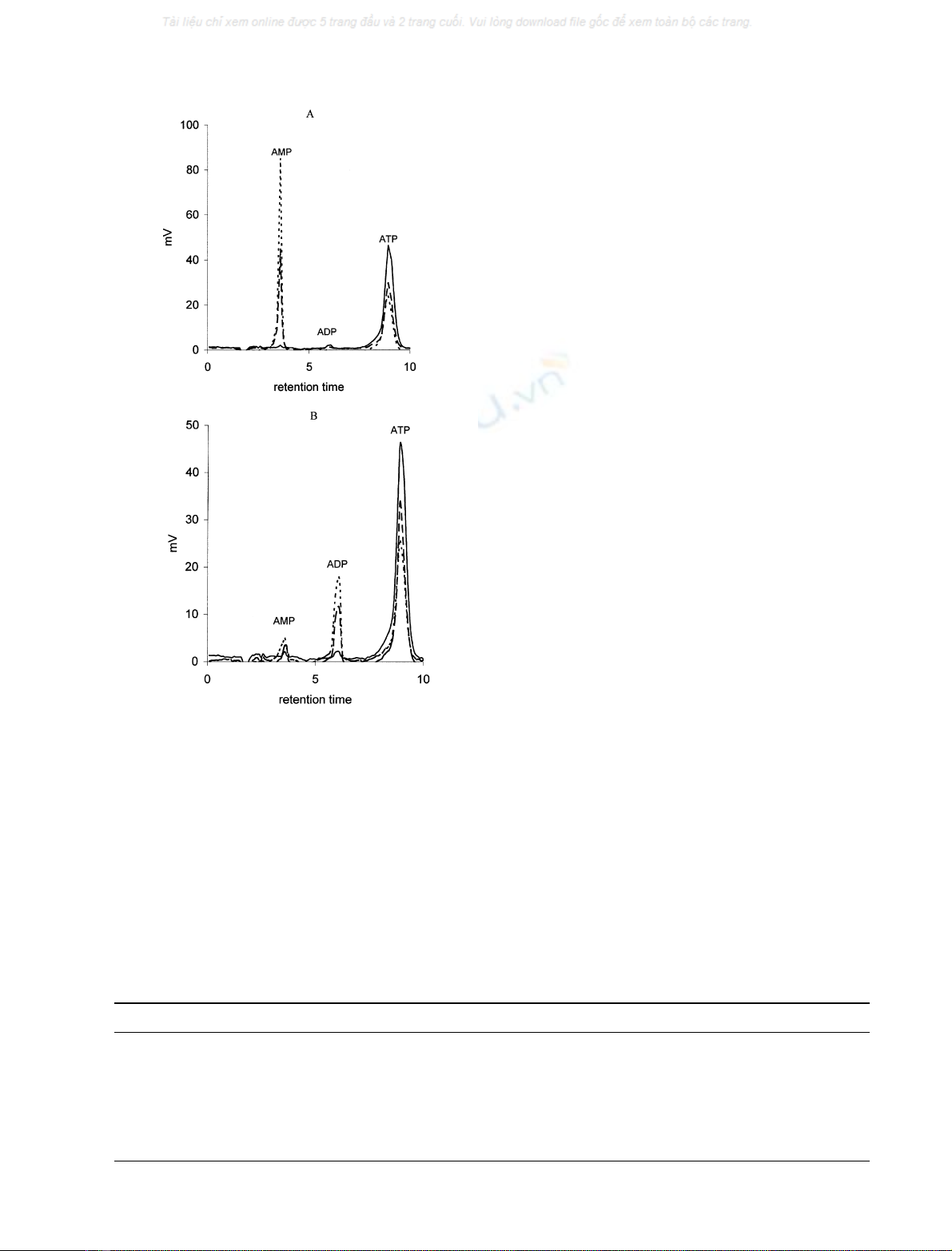
ATP to ADP and poorly ADP to AMP (ATP/ADP ratio
corresponded to 11.7) (Table 1, Fig. 4B). The protein eluted
from the column with 225 m
M
salt exhibited properties
characteristic for NTPDase1. This enzyme, with a ATP/
ADP ratio of 1.4 hydrolysed both ATP and ADP to AMP
(Table 1, Figs 3,4A). Apparent molecular weights of purified
ecto-nucleotidases during electrophoresis in SDS and
Western blotting were estimated to be 72 kDa for NTPD-
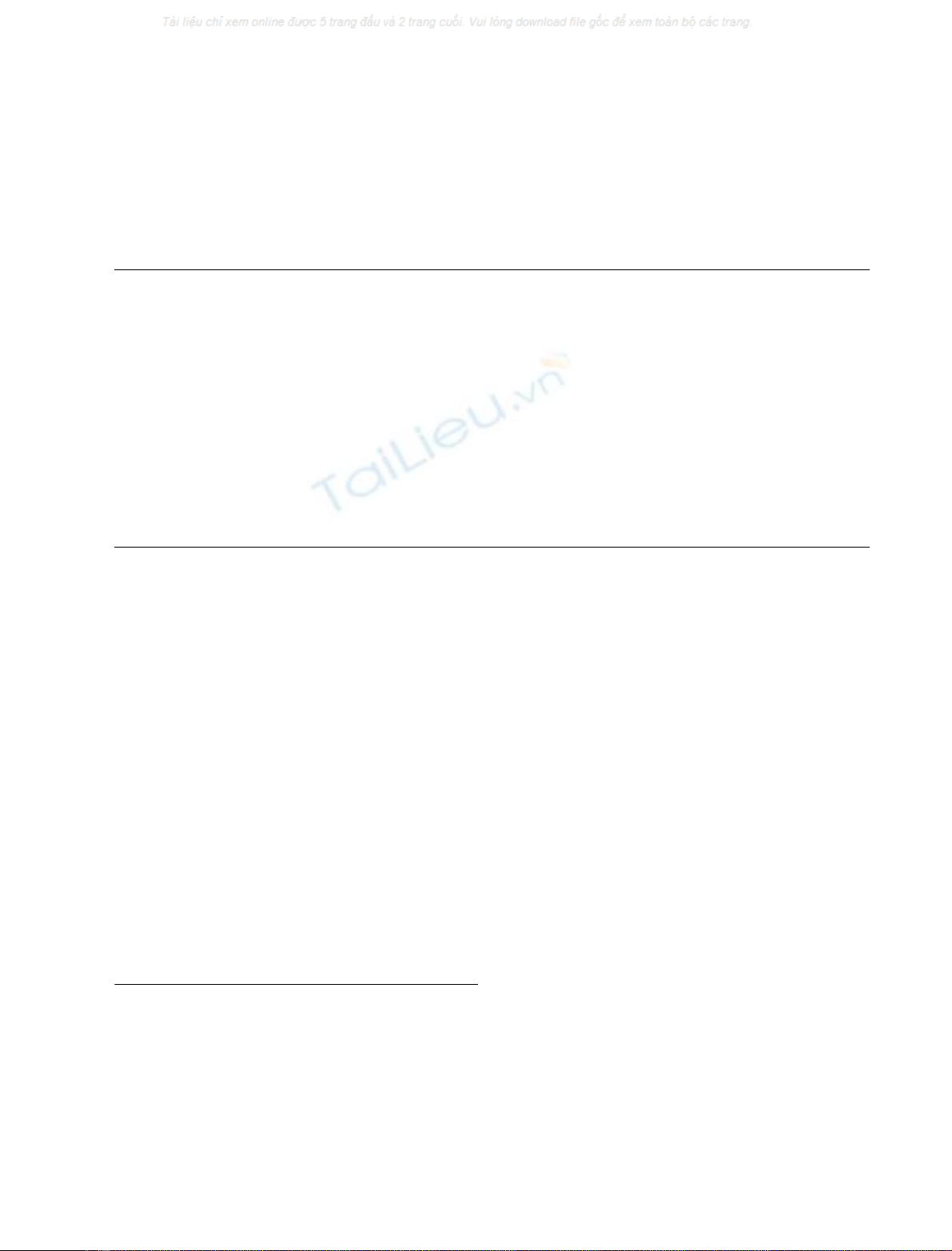
ase1 and 66 kDa for NTPDase2 (Fig. 2B).
Kinetic properties
Synaptosomal NTPDases differed slightly by isoelectric
point and pH optima (Table 2). Isoelectric points were
determined to be 5.1 for NTPDase1 and 5.4 for NTPDase2,
whereas the optimum pH value, was, respectively, 7.6 and
7.8 independently of tested substrates (ATP or ADP).
As expected, NTPDases from porcine synaptosomes
exhibited a high specificity towards pyrophosphate bonds
and low substrate specificity. They hydrolysed all purine
and pyrimidine nucleotides tested. NTPDase1 preferentially
hydrolysed ATP and UTP, whereas NTPDase2 UTP, TTP,
ITP and ATP. Both enzymes did not hydrolyse ester bonds
(Table 3). The purified hydrolases were activated by divalent
cations, however, EDTA did not inhibit their activity
completely (Table 4). Interestingly, ATP hydrolysis by
both enzymes was more effective with Mg
2+
and Mn
2+
ions, whereas ADP hydrolysis was more effective with
Ca
2+
ions. In the presence of Mg
2+
and Mn
2+
cations, the
ATP hydrolysis rate increased three times, whereas ADP
hydrolysis in the presence of these ions increased only 0.5
times (Table 4). Ca
2+
ions increased both ATP and ADP
hydrolysis by 2.5 times. The highest activation of NTPDases
by calcium ions was observed at 1.5 m
M
[Ca
2+
]. Cu
2+
and
Ba
2+
were strong inhibitors of the analysed enzymes,
whereas the presence of monovalent ions in the incubation
mixture did not affect the rate of ATP and ADP hydrolysis
(Table 4).
ATP and ADP were hydrolysed by synaptosomal
NTPDases in accordance with Michaelis–Menten kinetics.
Table 1. Purification of NTPDase1 and NTPDase2 from porcine brain cortex synaptosomes.
Purification
step
Total protein
(mg)
Total activity
(lmolÆP
i
Æmin
)1
)
Specific activity
(lmolÆP
i
Æmin
)1
Æ
mg of protien
)1
)
ATP/ADP
(ratio)
Purification
(-fold)ATP ADP ATP ADP
Synaptosomes 240 42 15 0.18 0.06 2.8 1.0
MonoQ–NTPDase1 0.04 2.6 1.8 69.4 49 1.4 777
a
MonoQ–NTPDase 0.004 0.35 0.03 79.6 6.7 12 455
b
a
In respect to ADP as substrate.
b
In respect to ATP as substrate.
Fig. 1. PAGE of NTPDase1 and NTPDase2 during different steps of
purification. Theproteinswereseparatedona5%acrylamidegel
containing 0.4% Triton X-100 and then silver stained. An amount of
protein (1–30 lg per well) was loaded. (A) Lane 1, polidocanol extract;
lane 2, NTPDase2 after chromatography on MonoQ column; lane 3,
NTPDase1 after chromatography on MonoQ column; lane 4,
NTPDase-enriched fraction after affinity chromatography on Con-A
Sepharose column; lane 5, NTPDases after molecular filtration.
(B) NTPDase activity in acrylamide gel was assayed in 50 mm Hepes
pH 7.4 containing 10 m
M
Ca
2+
and 1 m
M
ATP or ADP. In the spots
containing NTPDase activity white precipitates of calcium phosphate
were formed. Lanes 6 and 7, NTPDase activity with ADP and ATP as
substrates, respectively.
FEBS 2003 NTPDase1 and NTPDase2 from porcine synaptosomes (Eur. J. Biochem. 270) 3449
NTPDase1 had a similar K
m
for ATP and ADP
(97 ± 0.09 l
M
and 95 ± 0.13 l
M
, respectively; Table 2).
Affinity of ADP to NTPDase2 (K
m
¼5.5 ± 0.07 l
M
)was
more than 20 times lower than the affinity of ATP to this
enzyme (K
m
¼270 ± 1.2 l
M
) (Table 2). Despite the dif-
ferences in affinity of ATP of NTPDase1 and NTPDase2,
both enzymes possessed a similar molecular activity of 860
and 833 lmol productÆs
)1
, respectively. Simultaneously,
NTPDase1 characterized by a three times higher K
cat
/K
m
coefficient (9 ·10
6
M
)1
Æs
)1
) in comparison with the ana-
logous coefficient for NTPDase2 (3 ·10
6
)(Table2).
The activity of analysed enzymes was inhibited by
sodium azide, 8-butyl-thioATP and suramin, all known to
inhibit NTPDases [3,31–33]. ATPase inhibitors, e.g. oua-
bain, oligomycin and sodium orthovanadate, did not
change the rate of ATP and ADP hydrolysis. NTPDase
inhibition by azide was noncompetitive (data not shown).
NTPDase1 was more sensitive to azide than NTPDase2
(Table 5). Synaptosomal NTPDases were also noncom-
petitively inhibited by suramin, an antagonist of P2
receptors and simultaneously an inhibitor of ecto-ATPases
[2,31]. This compound decreased activity of analysed
hydrolases to a similar extent (Table 5). The strongest
inhibitor of both enzymes was BGO (Table 5), that was
shown previously to inhibit ATP/ADPase of bovine
spleen fractions [33]. This compound decreased the
activity of synaptosomal NTPDases competitively (data
not shown).
Purified ecto-hydrolases of pork brain synaptosomes in
contrast to ATP-diphosphohydrolase from rat brain cortex
synaptosomes [34], were insensitive to -SH group reagents,
i.e., NEM and p-chlorohydroksymercuric benzoic acid
(data not shown).
Fig. 2. SDS/PAGE and Western blot of NTPDase1 and NTPDase2
separated by ion-exchange chromatography on MonoQ column. NTP-
Dase1 and 2 obtained after ion-exchange chromatography were elec-
trophorized through 10% SDS/acrylamide gel and transferred onto a
nitrocellulose sheet. (A) An amount of 10 ng of protein was applied
per well and the proteins were silver stained. Apparent molecular
masses for NTPDase1 and NTPDase2 were estimated to be 72 and
66 kDa, respectively. Lane 1, molecular mass markers; lane 2,
NTPDase1; lane 3, NTPDase2. (B) Nitrocellulose sheet was incubated
with primary rabbit antibodies (RINGO) and then the bands were
visualized using alkaline phosphatase conjugated anti-rabbit Igs and
BCIP/NBT Sigma fast tablets as described. Lane, 4, molecular mass
markers; lane 5, NTPDase1; lane 6, NTPDase2.
Fig. 3. Chromatography of NTPDase1 and
NTPDase2 on MonoQ column.
3450 F. Kukulski and M. Komoszyn
´ski (Eur. J. Biochem. 270)FEBS 2003
Discussion
The presence of NTPDase1 and NTPDase2 in membranes
of brain cells was confirmed previously by analysis of a
cDNA library and Northern blot [5,7,10]. In the present
work, we have demonstrated the presence of both enzymes
in the synaptosomes of pig brain cortex. NTPDase1 and 2
were purified to homogeneity and revealed kinetic proper-
ties typical for representatives of this group of enzymes, i.e.,
low substrate specificity for tri- and diphosphonucleosides,
activation by divalent cations, and sensitivity to inhibitors
[3,8].
In various brain structures, receptors activated by ATP,
ADP, UTP and UDP occur simultaneously [2,35]. The
investigated enzymes hydrolysed effectively, not only ATP,
but also UTP and CTP, suggesting that they may partici-
pate in interruption of the signal transmitted by nucleotides
other than adenine. We observed the high activation of ATP
hydrolysis (over 300%) by Mg
2+
and Mn
2+
ions and low
activation of ADP hydrolysis by the same ions (less than
48%). In contrast, calcium ions stimulated the hydrolysis of
both substrates in equal degrees (200%). The majority of the
enzymes hydrolysing ATP prefer Mg
2+
–ATP complex as a
substrate [3]. NTPDase2 derived from chicken gizzard
smooth muscle was activated the most strongly by
Mg
2+
[36], whereas NTPDase1 from human placenta most
efficiently hydrolysed ATP in the presence of Ca
2+
[37].
Our results indicate that the complexes Mg
2+
–ATP or
Mn
2+
–ATP are preferred as substrate by synaptosomal
NTPDases, while in the case of ADP, the Ca
2+
–ADP
complexes are hydrolysed most effectively.
Results of previous examinations have indicated that
NTPDases show a high similarity in structure [4–6,10]. All
inhibitors used in our experiments changed the activity
of NTPDase1 and NTPDase2 to a similar extent. This
confirms that both purified NTPDases belong to the same
group of enzymes.
So far, the metabolic function fulfilled by NTPDase2 in
the synapse has not been explained. Homogeneity of the
obtained preparations allowed a precise analysis and
comparison of the kinetic properties of both enzymes. The
results of these experiments may help to define potential
roles of NTPDase1 and NTPDase2 in the neurotransmis-
sion mediated by nucleotides. We found that ATP has
three times higher affinity to NTPDase1 than to NTPDase2.
At the same time, both enzymes have similar molecular
activity. This indicates that under high substrate concen-
tration (close to V
max
for NTPDase2), the active sites of
both enzymes are fully saturated by ATP. Under such
conditions, both NTPDase1 and NTPDase2 will hydrolyse
ATP with a similar velocity. However, NTPDase1 has a
three times higher K
cat
/K
m
coefficient than NTPDase2. This
Fig. 4. Products of ATP hydrolysis in the reaction catalysed by purified
NTPDases. Reactions were carried out in the presence of 1 mm ATP.
Products of ATP degradation were analysed using HPLC RP. (A)
NTPDase1 hydrolysed ATP directly to AMP, (B) NTPDase2 hydro-
lysed ATP to ADP; (—–) control (– – –) after 10 min (- - - -) after 20 min.
Table 2. Physicochemical properties of purified NTPDases. Woolf–Augustinsson–Hofstee plot and
GRAPHPAD PRISM
softwarewereusedtoevaluate
K
m
and V
max
withATPandADPconcentrationfrom0.01to2m
M
. Molecular activity and K
cat
/K
m
coefficient were calculated for ATP as a
substrate. Values are expressed as mean ± SEM of three separate experiments, each conducted in triplicate.
Properties NTPDase1 NTPDase2
Optimum pH 7.6 7.8
Isoelectric point 5.1 5.4
K
m
values K
m(ATP)
¼97 ± 0.09 l
M
K
m(ATP)
¼270 ± 1.2 l
M
K
m(ADP)
¼95 ± 0.13 l
M
–
Molecular activity 860 lmol of product per s 833 lmol of product per s
K
cat
/K
m
coefficient 0.9 ·10
7
M
)1
Æs
)1
0.3 ·10
7
M
)1
Æs
)1
Apparent molecular mass 72 kDa 66 kDa
FEBS 2003 NTPDase1 and NTPDase2 from porcine synaptosomes (Eur. J. Biochem. 270) 3451


























