
Recruitment of coregulator complexes to the b-globin gene
locus by TFII-I and upstream stimulatory factor
Valerie J. Crusselle-Davis, Zhuo Zhou, Archana Anantharaman, Babak Moghimi, Tihomir Dodev,
Suming Huang and Jo
¨rg Bungert
Department of Biochemistry and Molecular Biology, Center for Mammalian Genetics, Shands Cancer Center, Powell Gene Therapy Center,
Genetics Institute, University of Florida, College of Medicine, Gainesville, FL, USA
Gene expression is regulated at multiple steps involv-
ing the relocation of genes in the nucleus, the modifi-
cation of chromatin structure and, ultimately, the
recruitment of transcription complexes [1–3]. Each step
is regulated by transcription factors that interact in a
sequence-specific manner with DNA control elements
located in promoters or enhancers [4]. These sequence-
specific binding proteins recruit coregulators that
modify histones, mobilize nucleosomes or recruit
components of the basal transcription machinery [5].
Tissue-specific genes are often regulated by tissue-
specific activators and repressors that act in concert
with ubiquitously expressed transcription factors.
The sequential stage-specific expression of the five
b-like globin genes is regulated by gene proximal
regulatory elements that recruit transcription factors
either activating or repressing gene expression [6].
The regulation of globin gene transcription involves
the recruitment of chromatin modifying activities
that regulate accessibility to subregions of the globin
gene locus in a developmental stage-specific manner.
High-level expression of the globin genes requires a
locus control region (LCR) located far upstream of the
embryonic e-globin gene and composed of five DNaseI
hypersensitive (HS) sites that are 200–400 bp in size
and separated form each other by 2–4 kbp [7–9]. The
LCR HS sites function together in a synergistic or
additive manner to stimulate globin gene expression
[10–12]. There is increasing evidence that transcription
of at least some, perhaps highly expressed, genes takes
Keywords
coregulator; globin genes; locus control
region; transcription
Correspondence
J. Bungert, Department of Biochemistry and
Molecular Biology, College of Medicine,
University of Florida, 1600 SW Archer Road,
PO Box 100245, Gainesville, FL 32610, USA
Fax: +1 352 392 2853
Tel: +1 352 273 8098
E-mail: jbungert@ufl.edu
(Received 16 May 2007, revised 3 October
2007, accepted 5 October 2007)
doi:10.1111/j.1742-4658.2007.06128.x
Upstream stimulatory factor and TFII-I are ubiquitously expressed helix-
loop-helix transcription factors that interact with E-box sequences and or
initiator elements. We previously demonstrated that upstream stimulatory
factor is an activator of b-globin gene expression whereas TFII-I is a
repressor. In the present study, we demonstrate that upstream stimulatory
factor interacts with the coactivator p300 and that this interaction is
restricted to erythroid cells expressing the adult b-globin gene. Further-
more, we demonstrate that Suz12, a component of the polycomb repressor
complex 2, is recruited to the b-globin gene. Reducing expression of Suz12
significantly activates b-globin gene expression in an erythroid cell line with
an embryonic phenotype. Suz12 also interacts with the adult b-globin gene
during early stages of erythroid differentiation of mouse embryonic stem
cells. Our data suggest that TFII-I contributes to the recruitment of the
polycomb repressor complex 2 complex to the b-globin gene. Together,
these data demonstrate that the antagonistic activities of upstream stimula-
tory factor and TFII-I on b-globin gene expression are mediated at least in
part by protein complexes that render the promoter associated chromatin
accessible or inaccessible for the transcription complex.
Abbreviations
ChIP, chromatin immunoprecipitation; EPO, erythropoietin; ES, embryonic stem; GAPDH, glyceraldehyde 3-phosphate dehydrogenase;
HDAC, histone deacetylase; HS, hypersensitive; LCR, locus control region; MEL, murine erythroleukemia; PRC, polycomb repressor
complex; siRNA, short interfering RNA; USF, upstream stimulatory factor.
FEBS Journal 274 (2007) 6065–6073 ª2007 The Authors Journal compilation ª2007 FEBS 6065

place in so-called transcription factories, specific
domains in the nucleus enriched for RNA polymer-
ase II and splicing factors [13,14]. A recent study by
Ragoczy et al. [15] demonstrates that the LCR medi-
ates the association of globin genes with transcription
factories during the differentiation of erythroid cells.
Observations that the LCR recruits transcription com-
plexes before they become detectable at globin gene
promoters support the hypothesis that the LCR is the
primary attachment site for the recruitment of macro-
molecular complexes involved in chromatin structure
alterations and transcription [16,17].
Regulation of the globin genes involves the action of
many transcription factors, some of which have been
characterized in detail. GATA-1, EKLF and NF-E2
are hematopoietic transcription factors that have all
been shown to participate in LCR function and b-glo-
bin gene expression [6]. In addition to these tissue
restricted transcription factors, ubiquitously expressed
transcription regulatory proteins such as Sp1, upstream
stimulatory factor (USF) and TFII-I have also been
demonstrated to regulate globin gene expression
[18,19]. Previous studies have shown that the helix-
loop-helix protein USF activates b-globin gene expres-
sion and interacts with E-box elements located in LCR
element HS2 and in the b-globin downstream pro-
moter region [20]. TFII-I, another helix-loop-helix pro-
tein, interacts with the b-globin initiator sequence and
represses b-globin gene expression. TFII-I exerts part
of its function by recruiting histone deacetylase
(HDAC) to the b-globin gene promoter and rendering
the chromatin inaccessible for transcription complexes
[20].
The polycomb repressor complex (PRC) has origi-
nally been identified in Drosophila, in which it plays an
important role in regulating the expression of segment
polarity genes, including the Hox gene cluster [21].
Homologous proteins have also been identified in
mammalian cells. There are two main PRC complexes,
PRC1 and PRC2. PRC2 contains the histone methyl-
transferase Ezh2, which methylates lysine 27 on the
histone H3 N-terminal tail [21]. This modification is
absent or reduced in promoters of transcribed genes.
PRC1 interacts with PRC2 and contains subunits that
recruit DNA methyltransferases. Current models pro-
pose that the PRC2 complex initially represses gene
activity by H3K27 methylation. Subsequent interac-
tions with the PRC1 complex appear to stabilize the
repressed chromatin structure by recruitment of DNA
methyl-transferases [22].
In the present study, we demonstrate that USF
interacts with coactivator p300 in mouse erythroleuke-
mia cells that express the b-globin gene. No inter-
actions are detectable between USF and p300 in K562
cells, a human erythroleukemia cell line that does not
express significant levels of the b-globin gene.
CBP ⁄p300 interacts with LCR element HS2 and the
b-globin promoter in murine erythroleukemia (MEL)
cells, but only with LCR HS2 in K562 cells. Further-
more, we demonstrate that the polycomb group pro-
tein Suz12 associates with the b-globin gene promoter
in K562 cells, but not in MEL cells. Inactivation of
Suz12 led to a three- to five-fold increase in b-globin
gene expression in K562 cells. Our data suggest that
TFII-I recruits HDAC3 and the PRC2 complex to the
b-globin gene promoter to establish an inaccessible
chromatin configuration.
Results
CBP
⁄
p300 interacts with USF and the b-globin
promoter in MEL but not in K562 cells
We have shown previously that USF is required for
high-level b-globin gene expression in MEL cells [20].
USF functions as a classical transcription factor that is
able to stimulate transcription in in vitro transcription
systems [23]. Recent data from the Felsenfeld labora-
tory have shown that USF is also a critical part of a
chromatin boundary in the chicken b-globin gene locus
[24]. It was shown that USF interacts with CBP ⁄p300,
suggesting that it can function at least in part by
recruiting chromatin modifying activities. MEL cells
expressing a dominant negative mutant of USF exhib-
ited a reduction in Pol II loading to LCR element HS2
and to the b-globin gene promoter [20]. At the same
time, we observed a reduction in the recruitment of
CBP and p300 to these sites.
To examine in more detail whether USF recruits co-
activators to the b-globin gene locus, we first examined
the recruitment of different coactivators to regions in
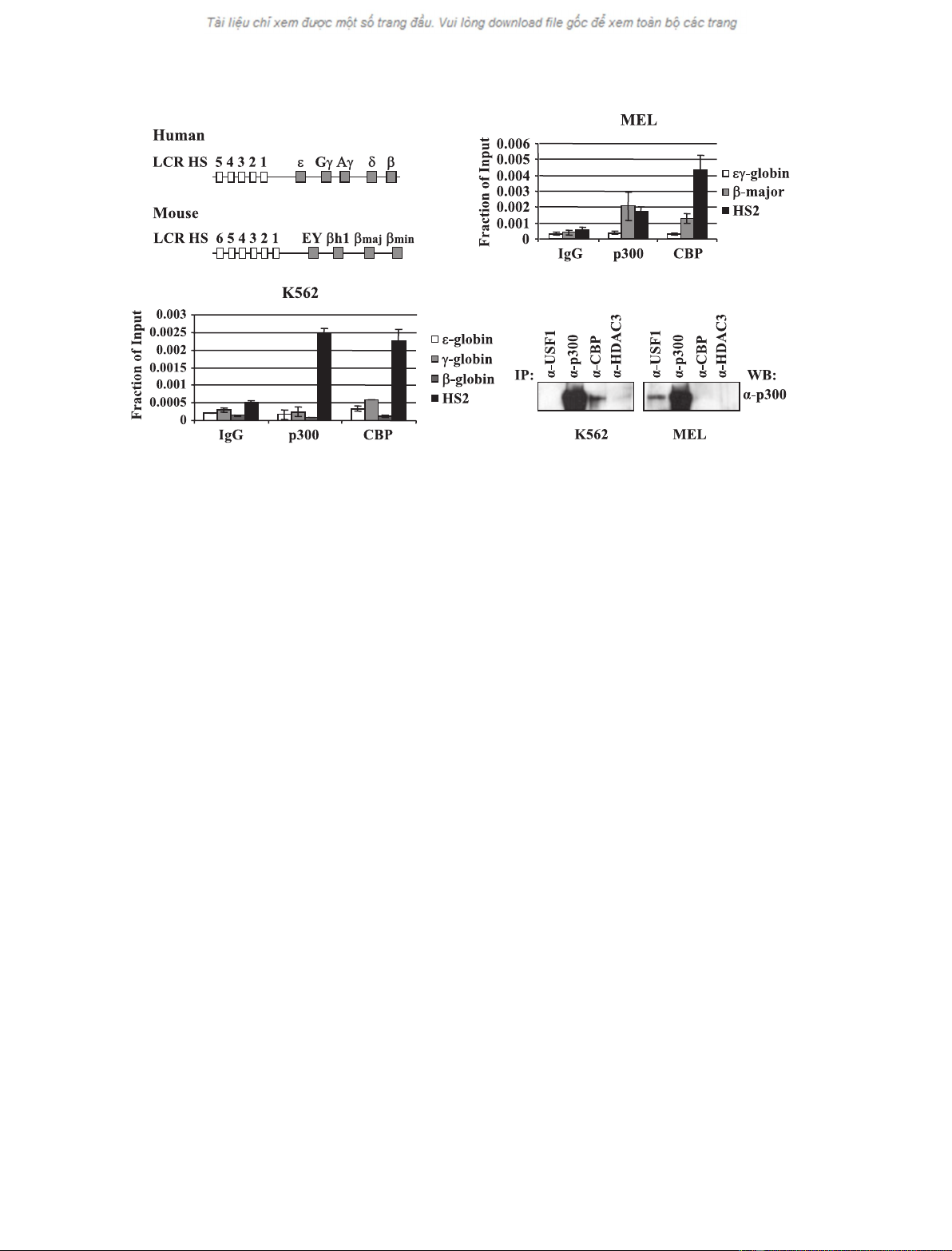
the globin gene locus (Fig. 1A). The data show that
CBP and p300 are efficiently crosslinked at the tran-
scribed b-globin gene promoter and at LCR element
HS2 in MEL cells (Fig. 1B). No interactions were
detected at the repressed embryonic ec-globin gene
promoter (Fig. 1B). In K562 cells, CBP and p300
interact efficiently with HS2 but not with the repressed
b-globin gene promoter (Fig. 1C). There is some inter-
action between CBP and the expressed e- and c-globin
genes in K562 cells but the interaction appears to be
less efficient compared to LCR HS2.
We next analyzed interactions between USF and p300
in MEL and K562 cells by co-immunoprecipitation
(Fig. 1D). The results demonstrate that USF interacts
with p300 in MEL cells but not in K562 cells. This
Coregulators of USF and TFII-I in erythroid cells V. J. Crusselle-Davis et al.
6066 FEBS Journal 274 (2007) 6065–6073 ª2007 The Authors Journal compilation ª2007 FEBS
interaction is specific because no interactions between
HDAC3 and p300 are observed. Taken together, the
data demonstrate that USF interacts with the coactiva-
tor p300 in erythroid cells and suggest that it recruits
p300 to specific regions in the b-globin gene locus.
Interaction of Suz12 with the b-globin gene
promoter in K562 but not in MEL cells
TFII-I interacts with the b-globin initiator and
represses b-globin gene expression in embryonic
erythroid cells [20]. We have shown previously that
TFII-I interacts with HDAC3 in K562 cells. Polycomb
group proteins were originally identified as repressors
of gene expression during development in Drosophila.
Recently, it was shown that the PRC2 is located at
and represses developmentally regulated genes in
undifferentiated, embryonic stem (ES) cells [22]. To
examine whether PRCs are located at the b-globin
gene promoter in embryonic cells, we carried out chro-
matin immunoprecipitation (ChIP) experiments using
antibodies against Suz12, a component of PRC2, in
K562 and MEL cells (Fig. 2). The data demonstrate
that Suz12 can be crosslinked to the repressed b-globin
gene promoter in K562 cells (Fig. 2A) but not to the
transcribed b
maj
-globin gene promoter in MEL cells
(Fig. 2B). We did not detect any interactions of Suz12
with the embryonic e-globin gene promoter. The Suz12
antibody used in these experiments specifically recog-
nizes both mouse and human proteins and Suz12 is
expressed in both K562 and MEL cells as determined
in western blotting experiments (data not shown).
Interaction of TFII-I with HDAC3 and Suz12
We previously demonstrated that TFII-I interacts with
HDAC3 and recruits this protein to the b-globin gene
promoter in K562 cells. In the present study, we ana-
lyzed the interaction between TFII-I and HDAC3 in
both MEL and K562 cells and show that this inter-
action is restricted to K562 cells. We next wished to
examine whether the PRC2 complex, which interacts
with the b-globin gene in K562 cells, could be recruited
to the gene by TFII-I. Co-immunoprecipitation experi-
ments demonstrate that TFII-I interacts with HDAC3
in K562 cells, consistent with our previous data, but
AB
CD
Fig. 1. CBP ⁄p300 interact with the b-globin gene locus and with USF1 in MEL cells but not in K562 cells. (A) Schematic of the organization
of the human and mouse b-globin gene loci. The human b-globin locus depicted on top consists of five genes which are expressed in a
developmental stage-specific manner in erythroid cells as outlined. The expression of the genes is regulated by a LCR composed of five
DNaseI HS and located approximately 15–27 kbp upstream of the embryonic e-globin gene. The murine b-globin gene locus, which is
depicted on the bottom, consists of four genes which are expressed either in erythroid cells of the embryonic yolk sac (EY or bH1) or in
definitive erythroid cells derived from fetal liver or bone marrow hematopoiesis (b
maj
and b
min
). The murine LCR also contains multiple HS
required for high-level globin gene expression. K562 (B) and MEL cells (C) were subjected to ChIP analysis with antibodies against p300,
CBP or with a nonspecific antibody (IgG). Purified DNA was analyzed by real-time quantitative PCR with primers specific for LCR HS2 or the
e-, c-, b-, b-major or ec-globin gene promoters. (D) K562 or MEL cell extracts were precleared with anti-(rabbit IgG) beads and precipitated
with a-USF1, a-p300, a-CBP or a-HDAC3 (as negative control), and complexes were captured by incubation with anti-(rabbit IgG) beads.
Complexes were eluted off the beads with Laemmli buffer and incubation at 95 C for 10 min and loaded onto a 5% Ready gel (Bio-Rad).
The membrane was probed with a-p300.
V. J. Crusselle-Davis et al.Coregulators of USF and TFII-I in erythroid cells
FEBS Journal 274 (2007) 6065–6073 ª2007 The Authors Journal compilation ª2007 FEBS 6067
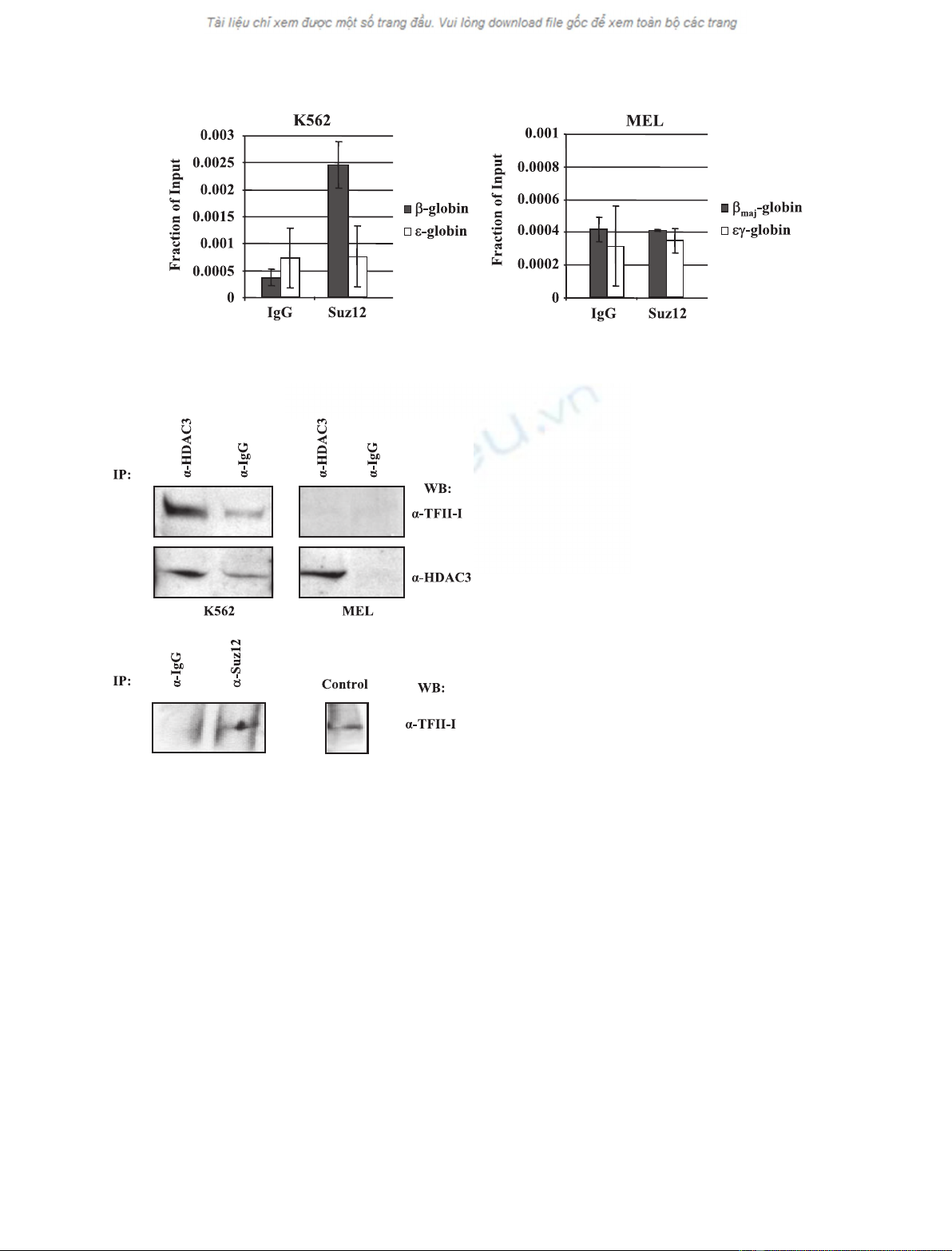
not in MEL cells, in which the b-globin gene is tran-
scribed (Fig. 3A). We also detected interactions
between TFII-I and Suz12 in K562 cells (Fig. 3B). The
interaction between TFII-I and Suz12 is not as efficient
as that involving HDAC3 and it is not restricted to
K562 cells, because interactions are also detectable in
MEL cells (data not shown).
Reduction of Suz12 expression in K562 cells
increases b-globin gene expression
We previously used SMART-pool short interfering
RNA (siRNA) reagent from Dharmacon and effi-
ciently reduced expression of TFII-I and HDAC3 [20].
Reductions in both TFII-I and HDAC3 expression by
more than 80% led to an approximately three-fold
increase in b-globin gene expression. To examine
whether Suz12 and the PRC2 complex participate in
the repression of the adult b-globin gene in K562 cells,
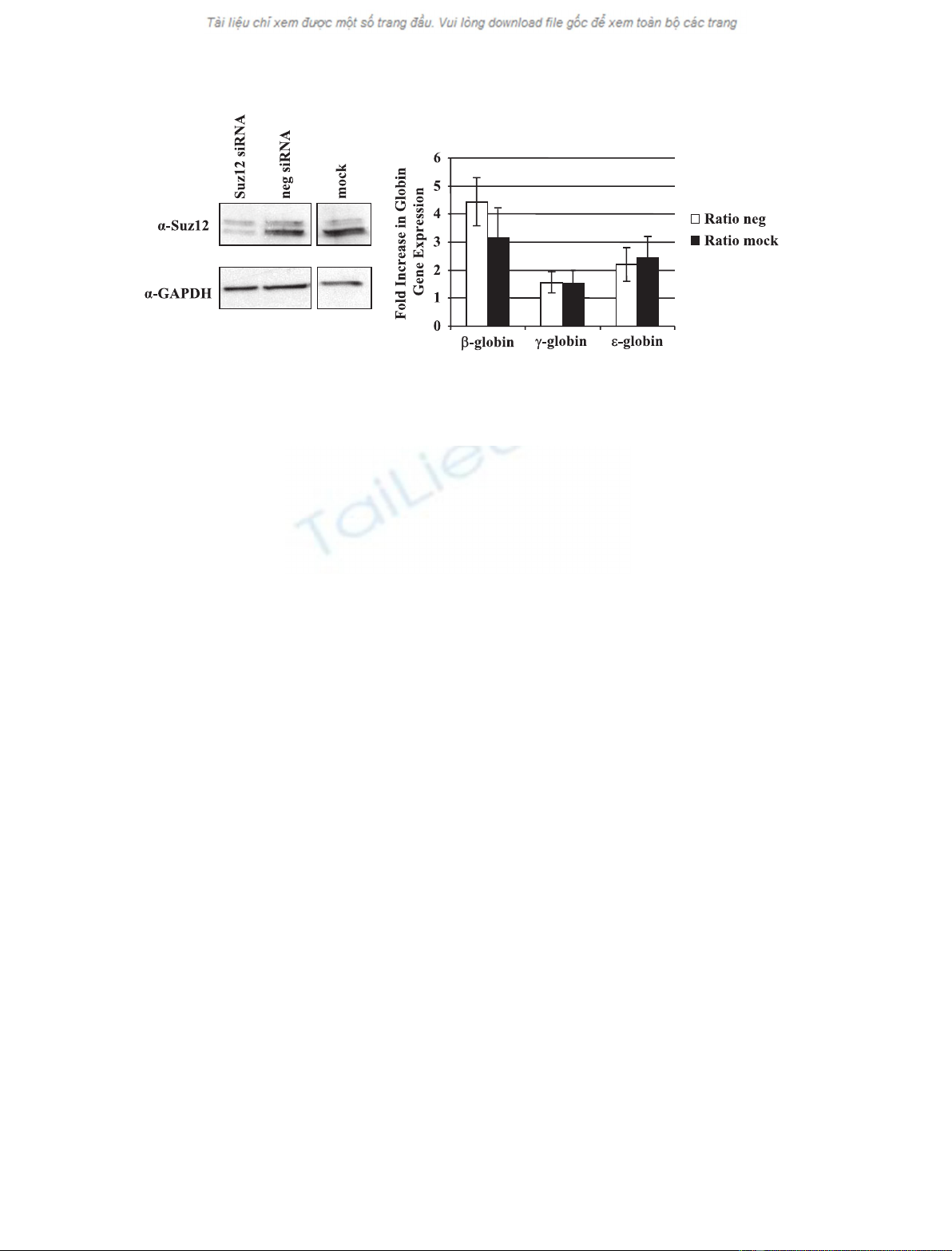
we reduced expression of Suz12 by RNA interference.
The western blot results demonstrate that siRNA
transfected cells reveal a drastic reduction in Suz12
protein levels compared to mock transfected cells or
cells transfected with nonspecific siRNA (Fig. 4A).
Expression of the adult b-globin gene was increased by
three- to five-fold in cells transfected with Suz12
siRNA compared to mock or negative control siRNA
transfected cells (Fig. 4B). These results demonstrate
that the PRC2 complex, or components thereof, partic-
ipate in the repression of the adult b-globin gene in
embryonic erythroid cells. We also detected an increase
in the expression of the embryonic e-globin gene.
However, this increase was not as pronounced as the
one seen for b-globin gene expression.
The PRC2 complex contains the histone H3K27
specific histone methyltransferase Ezh2 [21]. Ezh2
catalyzes the di- and tri-methylation of H3K27 [25].
Using ChIP, we did not detect high levels of tri-
methylated H3K27 at the globin gene locus in K562
cells, consistent with studies from the Blobel labora-
AB
Fig. 2. Suz12 interacts with the adult b-globin gene in K562 but not in MEL cells. Antibodies against Suz12 and nonspecific IgG were used
in ChIP assays using K562 (A) and MEL (B) cells. Quantitative PCR was performed with primers that amplified the promoters of the genes
as indicated.
A
B
Fig. 3. Interaction of Suz12 and HDAC3 with TFII-I. (A) Interaction
of HDAC3 with TFII-I in K562 but not MEL cells. K562 or MEL cell
extract was precleared with anti-(a)-(rabbit IgG) beads and precipi-
tated with a-HDAC3 or IgG, and complexes were captured by incu-
bation with anti-(rabbit IgG) beads. Complexes were eluted off the
beads with Laemmli buffer and incubation at 95 C for 10 min and
loaded onto a 10% Ready gel (Bio-Rad). The membrane was
probed with a-TFII-I and then stripped and probed with a-HDAC3
as a positive control. (B) Interaction of Suz12 with TFII-I in K562
cells. K562 extracts were precleared with anti-(rabbit IgG) beads
and precipitated with 2.5 lgofa-Suz12 or 2.5 lgofa-IgG (as nega-
tive control) antibodies. The complexes were captured by incuba-
tion with anti-(rabbit IgG) beads. Complexes were eluted off the
beads with Laemmli buffer and incubation at 95 C for 10 min and
loaded onto a 10% Ready gel (Bio-Rad). The membrane was
probed with a-TFII-I. The lane labeled control represents a regular
western blot for TFII-I with protein extract from K562 cells.
Coregulators of USF and TFII-I in erythroid cells V. J. Crusselle-Davis et al.
6068 FEBS Journal 274 (2007) 6065–6073 ª2007 The Authors Journal compilation ª2007 FEBS
tory [26]. In addition, the association of trimethylated
H3K27 was not significantly altered in cells that
express, or do not express, the adult b-globin gene
(data not shown).
Interaction of Suz12 with the b
maj
-globin gene
promoter decreases during activation of the
b
maj
-globin gene in differentiating murine ES cells
We next examined the association of Suz12 with the
b
maj
-globin gene promoter during differentiation of
mouse ES cells. We previously demonstrated that the
adult b-globin gene is expressed at low levels in ES
cell cultures incubated for 5 days with erythropoietin
(EPO), which mediates the differentiation and prolif-
eration of erythroid cells [17]. High-level expression
of the adult b-globin gene was observed at day 12 in
the ES cell differentiation system. Figure 5A,B dem-
onstrates that b
maj
-globin gene expression is up-regu-
lated by more than 30-fold between days 5 and 12.
We observed that the association of Suz12, TFII-I
and trimethylated H3K27 (H3K27me3) with the
b
maj
-globin gene promoter is high at day 5 but
undetectable at day 12 (Fig. 5C). Quantitation of the
Suz12 levels at the b
maj
-globin gene promoter dem-
onstrate that the changes between days 5 and 12 are
significant. The control experiment demonstrates that
interaction of LCR HS2 associated dimethylated
H3K4 (H3K4me2) does not change during the
course of differentiation. Neither Suz12, nor TFII-I
were found to associate with a control region located
between LCR elements HS2 and HS3 (data not
shown).
Discussion
We provide evidence that USF and TFII-I regulate
b-globin gene expression through the recruitment of
coactivator complexes that render the b-globin pro-
moter accessible or inaccessible to the transcription
complex. USF recruits the histone acetyltransferase
p300 to the b-globin promoter and this activity
increases the accessibility for transcription factors.
TFII-I recruits HDAC3 and the PRC2 complex, which
render the chromatin structure inaccessible to the tran-
scription complex.
Previous data from the Felsenfeld laboratory have
shown that USF interacts with the coregulators p300,
CBP, SET7 ⁄9 and PCAF and perhaps recruits these
activities to a chromosomal boundary element [24]. In
the present study, we show that p300 and CBP are
located at the promoter of the active adult b-globin
gene and also at LCR element HS2. USF1 is observed
to interact with p300 exclusively in erythroid cells with
an adult phenotype. These data suggest that USF1
recruits p300 to the promoter of the active b-globin
gene to aid in transcriptional activation.
CBP ⁄p300 is located at LCR HS2 in K562 cells, in
which we did not detect interactions between USF and
p300. This suggests that the recruitment of CBP ⁄p300
to the LCR, at least in K562 cells, is mediated by pro-
teins other than USF, and potential candidates are
GATA-1 and NF-E2, which have been shown to inter-
act with CBP [27,28]. Both of these proteins were
shown to be required for histone acetylation of spe-
cific regions in the globin gene locus. However, the
recruitment of CBP ⁄p300 to the adult b-globin gene
AB
Fig. 4. Suz12 represses b-globin gene expression in K562 cells. K562 cells were nucleofected with Suz12 siRNA, nontargeting siRNA (neg),
or mock transfected. (A) Protein was collected after 2 days and electrophoresed on gels for western blotting. Blots were probed with
a-Suz12 in the upper panel and then stripped and reprobed with a-GAPDH for a loading control. (B) Relative b-, c- and e-globin expression in
Suz12 knockdown cells. RNA was collected, reverse transcribed, and analyzed by quantitative PCR. Expression is set relative to either non-
targeting siRNA (neg) samples or mock transfected cells, with GAPDH as the internal reference.
V. J. Crusselle-Davis et al.Coregulators of USF and TFII-I in erythroid cells
FEBS Journal 274 (2007) 6065–6073 ª2007 The Authors Journal compilation ª2007 FEBS 6069


























