
Synergistic co-operation of signal transducer and
activator of transcription 5B with activator protein 1
in angiotensin II-induced angiotensinogen gene activation
in vascular smooth muscle cells
Mei Han, Ai-Ying Li, Fang Meng, Li-Hua Dong, Bin Zheng, Hai-Juan Hu, Lei Nie and Jin-Kun Wen
Department of Biochemistry and Molecular Biology, Hebei Medical University, Shijiazhuang, China
Angiotensin II (Ang II), an extensively characterized
peptide produced by successive proteolytic cleavage
reactions of its prohormone, angiotensinogen (AGT),
is an important contributor to the regulation of vol-
ume homeostasis and blood pressure in humans and to
the initiation of pathophysiological events that lead to
hypertension and cardiovascular disorders [1,2].
In human genetic studies, a clear linkage has been
established between the AGT gene and hypertension
[3]. Several lines of evidence have indicated that small
variations in AGT concentration result in substantial
changes in the circulating Ang II levels [4]. At the
cellular level, Ang II-mediated signaling is achieved
through its binding to the cell-surface AT1 receptor,
which causes activation of Janus kinase 2 (JAK2) [5,6]
and then activates signal transducer and activator of
transcription (STAT) molecules in cardiac myocytes
and in rat aortic (vascular) smooth muscle cells
(VSMCs) [5,7–9], resulting in the positive feedback of
AGT transcription [5]. The AGT gene itself is the
target for the activated STAT protein in cardiac myo-
cytes through the AGT promoter region [5]. However,
the interaction of STAT5B with the AGT gene
promoter was observed in liver and cardiac myocytes
[8,10], but not in the smooth muscle cell line.
The molecular basis for activation of the AGT gene
is only partially understood. The analysis of biological
information presumes that the 500-bp region of the rat
Keywords
activator protein-1; angiotensinogen; gene
regulation; signal transducer and activator of
transcription-5; vascular smooth muscle
cells
Correspondence
J.-K. Wen, Department of Biochemistry and
Molecular Biology, No. 361, Zhongshan East
Road, Shijiazhuang 050017, China
Fax: +86 311 8626 6180
Tel: +86 311 8626 5563
E-mail: wjk@hebmu.edu.cn
(Received 29 October 2008, revised 29
December 2008, accepted 12 January 2009)
doi:10.1111/j.1742-4658.2009.06902.x
The binding sequences for signal transducer and activator of transcription
(STAT) and activator protein 1 have been found in the promoter region of
the angiotensinogen gene. We examined whether the elements for activator
protein 1 and STAT5B function in angiotensinogen gene activation induced
by angiotensin II in vascular smooth muscle cells. Stimulation with angio-
tensin II increased the level of angiotensinogen mRNA by 2.1-fold in
vascular smooth muscle cells. The increased level of angiotensinogen
mRNA occurred with concurrent elevations in the levels of STAT5B and
c-Jun phosphorylation after stimulation with angiotensin II. Likewise,
angiotensin II resulted in similar enhancements of the DNA-binding activ-
ity of STAT5B and c-Jun in angiotensin II-induced angiotensinogen expres-
sion. Notably, the STAT5B–DNA complex interacted with the c-Jun–DNA
complex by forming a stable quaternary complex in angiotensin II-induced
angiotensinogen expression. Our findings support a model in which
co-operative interaction of STAT5B and activator protein 1 bound to the
the promoter region provides maximal activation of angiotensinogen
expression by angiotensin II in vascular smooth muscle cells.
Abbreviations
AGT, angiotensinogen; Ang II, angiotensin II; AP-1, activator protein 1; ChIP, chromatin immunoprecipitation; CoIP, cross-
coimmunoprecipitation; EMSA, electrophoretic mobility shift assay; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; JAK, Janus kinase;
STAT, signal transducer and activator of transcription; VSMCs, vascular smooth muscle cells.
1720 FEBS Journal 276 (2009) 1720–1728 ª2009 The Authors Journal compilation ª2009 FEBS

AGT gene promoter contains clusters of regulatory
elements, perfectly or partially matched to consensus
sequences, including binding sequences for STAT5B
and activator protein 1 (AP-1). Previous studies indi-
cate that transcription activation by STATs requires
activated AP-1 [11–13]. AP-1 is a complex composed
of the Fos and Jun proteins [14–16]. In general, Fos
and Jun family proteins function as dimeric transcrip-
tion factors that bind to AP-1 regulatory elements in
the promoter and enhancer regions of the target
[14,16]. However, the role of AP-1 in AGT gene tran-
scription activation is unknown. It has been demon-
strated that STAT proteins co-operate to bind to
target DNA, not only with other STAT family mem-
bers [17–19], but also with other proteins and tran-
scription factors [11,13,20,21]. Recently, the physical
association between STAT and c-Jun on the a
2
-macro-
globulin promoter element has been shown to yield
maximal enhancer function [13].
Based on these pieces of knowledge, we hypothe-
sized that co-operative interaction between c-Jun and
STAT5B may be important in transcription activation
of the AGT gene induced by Ang II. To understand
whether elements for AP-1 and STAT5B function in
AGT gene activation induced by Ang II, we tested the
effect of co-operative interaction between c-Jun and
STAT5B on the AGT promoter activity and AGT
mRNA expression in VSMCs. We showed that
Ang II-induced AGT expression in VSMCs involves
co-operation between AP-1 and STAT5B. We also
demonstrated that there exists a physical interaction
between AP-1 and STAT5B during AGT expression
induced by Ang II.
Results
Ang II increases AGT gene expression with
concurrent increases in the phosphorylation
of STAT5B and c-Jun in VSMCs
It has been demonstrated that Ang II stimulates
AGT expression in hepatocytes [22] and in cardiac
muscle [5]. The present study showed that Ang II
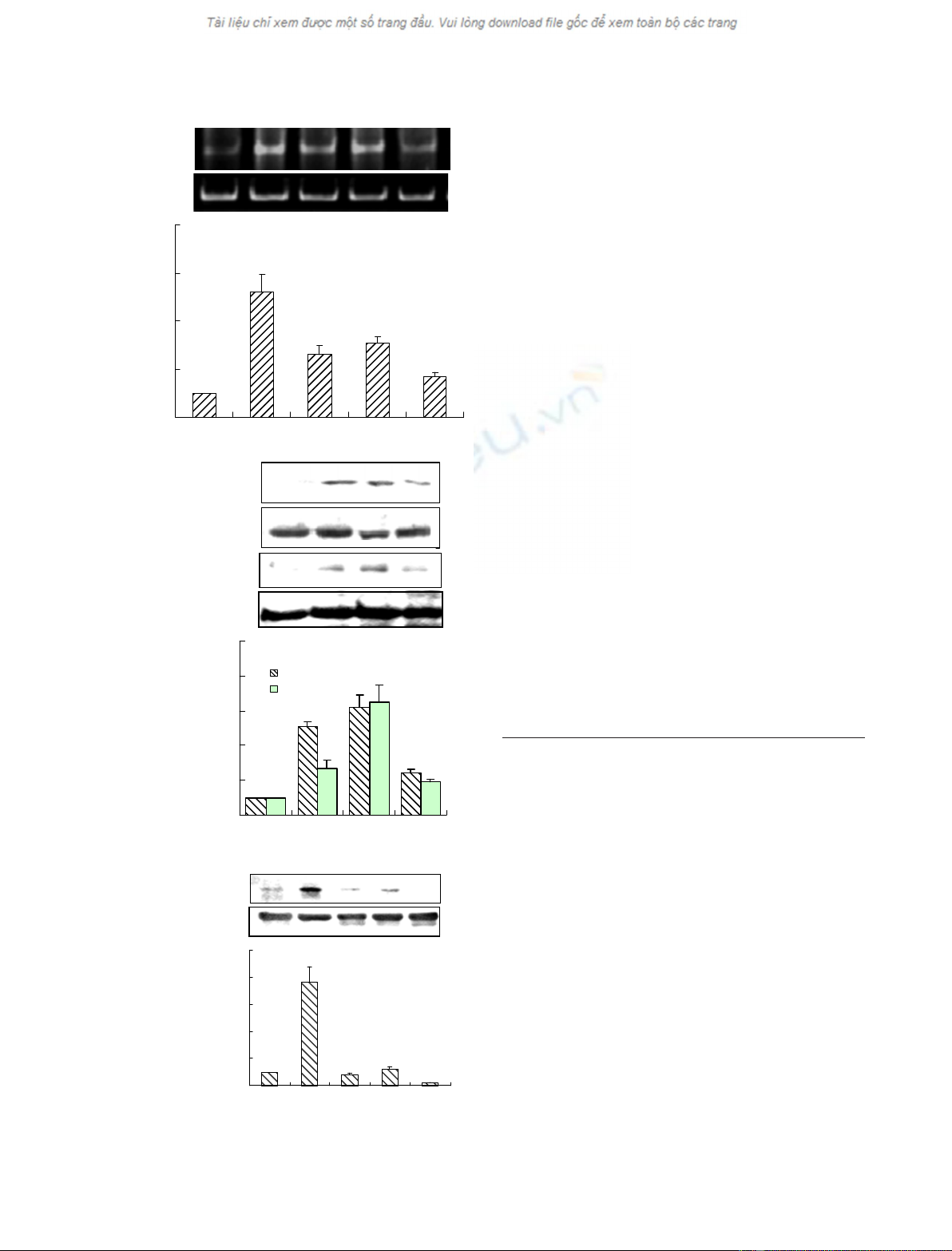
increased the AGT mRNA level in VSMCs. Follow-
ing treatment of VSMCs with Ang II for different
periods of time, AGT mRNA was detected using
RT-PCR. As shown in Fig. 1A, the level of AGT
mRNA peaked 3 h after stimulation with Ang II,
showing an increase of 5.2-fold, and decreased there-
after. STAT5B is necessary for expression of the
AGT gene [10], and Ang II activates JAK-STAT
and AP-1 [23]. To determine the relationship
between the activation of STAT5B and c-Jun, and
the expression of the AGT gene in VSMCs stimu-
lated with Ang II, the effect of Ang II on the phos-
phorylation of STAT5 and c-Jun was measured.
Ang II stimulated the phosphorylation of STAT5B
and c-Jun, with levels of phosphorylated STAT5B
and c-Jun significantly increasing 1 h, and peaking
3 h, after stimulation with Ang II, whereas total
c-Jun and STAT5B were not changed after treatment
with Ang II for different periods of time (Fig. 1B).
However, the phosphorylation of STAT5B induced
by Ang II was dramatically inhibited by pretreating
VSMCs with AG490 (a specific inhibitor of the
JAK-STAT pathway) for 16 h [24], indicating that
the activation of STAT5B and c-Jun may be
involved in Ang II-induced AGT mRNA expression
(Fig. 1C).
DNA-binding activity of STAT5B and c-Jun
increases in Ang II-induced AGT expression
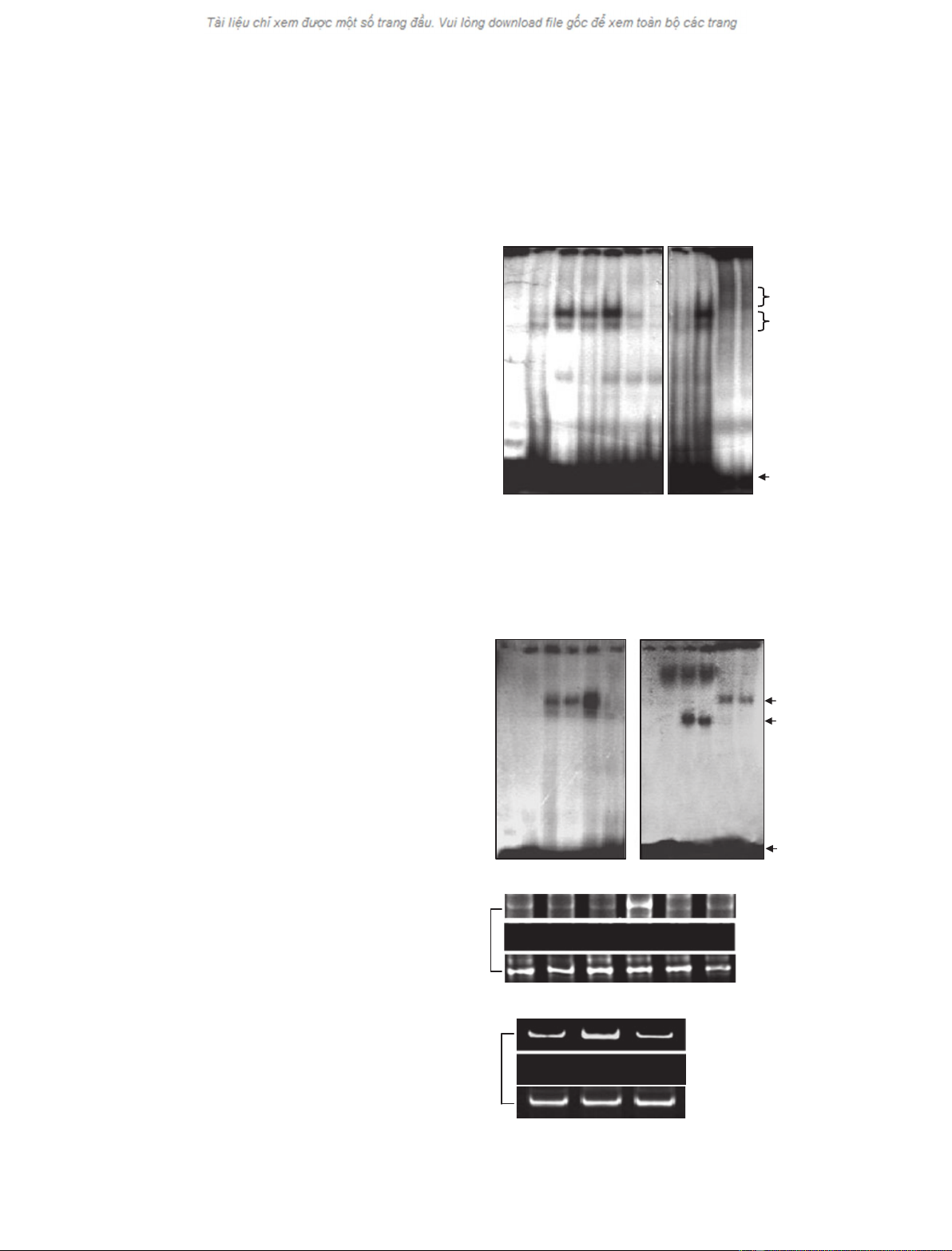
To find out whether the increase in STAT5B and
c-Jun phosphorylation induced by Ang II affects the
binding of STAT5B and c-Jun to their cis-elements,
the activity of STAT5B binding and of c-Jun binding
to DNA was detected, respectively, by electrophoretic
mobility shift assays (EMSAs) using radiolabeled oli-
gonucleotides containing either a STAT5B-binding site
or an AP-1-binding site in the rat AGT gene promoter.
As shown in Fig. 2A,B, DNA–protein complexes were
formed when these two probes were incubated with
nuclear extracts from VSMCs treated with Ang II for
0.5, 1, and 3 h, and the DNA-binding activity of
STAT5B and of AP-1 increased in a time-dependent
manner. The specificities of two DNA–protein com-
plexes were demonstrated by their disappearance upon
the addition of a 100-fold molar excess of unlabeled
probe. Further to confirm whether STAT5B and AP-1
are involved in the shifted complexes, supershift assays
were performed by adding antibodies against STAT5B
or c-Jun. Figure 2A,B showed new supershifted bands,
indicating that the complexes contained STAT5B or
c-Jun. Finally, to verify whether Ang II can stimulate
recruitment of STAT5B and c-Jun to the AGT pro-
moter in vivo, chromatin immunoprecipitation (ChIP)
assays were performed using antibodies to STAT5B
and to c-Jun, respectively. As shown in Fig. 2C, DNA
fragments containing the STAT5B- and AP-1-binding
sites could be detected in the immunoprecipitates
pulled by anti-c-Jun or anti-STAT5B IgGs. Increased
binding of AP-1 or STAT5B to the AGT promoter
was observed in VSMCs treated with Ang II for 3 h.
However, AG490 decreased the recruitment of
STAT5B to the AGT promoter region with the
M. Han et al. STAT5B and AP-1 interaction in AGT gene activation
FEBS Journal 276 (2009) 1720–1728 ª2009 The Authors Journal compilation ª2009 FEBS 1721
inhibition of STAT5B phosphorylation (Fig. 1C and
Fig. 2C), suggesting that STAT5B phosphorylation is
necessary for its binding to the AGT promoter.
Co-operation of STAT5B with AP-1 activates the
AGT promoter
To determine whether the binding of STAT5B and
AP-1 with the AGT promoter is essential to AGT
expression induced by Ang II, 293A cells were cotrans-
fected with the pGL3-AGT-Luc reporter plasmid, which
contains both AP-1- and STAT5B-binding sequences in
the AGT promoter from )545 to 39 bp (Fig. 3A), and
pcDNA3.1-STAT5B and ⁄or pcDNA3.1-c-Jun expres-
sion plasmids. Overexpression of STAT5B or c-Jun
alone modestly increased the reporter activity following
stimulation with Ang II (Fig. 3B). On the other hand,
the cotransfection of STAT5B with c-Jun expression
vectors significantly increased the AGT reporter activity
by 6.8-fold over that seen with the reporter alone. These
results indicate that STAT5B and c-Jun synergistically
activate AGT gene transcription.
STAT5B and AP-1 form a stable complex in the
AGT promoter in Ang II-induced AGT expression
To establish whether there is a direct interaction
between STAT5B and c-Jun in the expression of AGT
induced by Ang II stimulation, DNA–protein inter-
actions were investigated by cross-supershift assays. As
seen in EMSAs, DNA–protein complexes formed by
0
2
4
6
8
10
0 h 1 h 3 h 6 h
Relative level
p-c-Jun
pSTAT5B
Ang II 0136 h
IP: c-Jun/ IB: p-Ser
IP:c-Jun/ IB: c-Jun
IP:STAT5B/IB: PY99
*
*
*
*
*
*
Ang II
A
B
C
03 61224 h
AGT
GAPDH
*
*
*
*
Relative mRNA level
0
2
4
6
8
0 h 3 h 6 h 12 h 24 h
IP: STAT5B/IB: PY99
IP:STAT5B/IB: STAT5B
Ang II –3366 h
AG490 –– + +–
–3366 h
––+ +–
*
0
2
4
6
8
10
Relative pSTAT5B level
Ang II
AG490
*
IP:STAT5B/IB: STAT5B
Fig. 1. Ang II induces AGT gene expression with concurrent
increases in the phosphorylation of c-Jun and STAT5B in VSMCs.
(A) VSMCs were treated with Ang II (10
)7
M) for 0, 3, 6, 12 and
24 h. Total RNA was isolated from VSMCs and subjected to
RT-PCR analysis using specific primers of the AGT gene. GAPDH
was used as an internal control. Bar graphs show the relative level
of AGT mRNA for four independent experiments. *P< 0.05, com-
pared with 0 h (n= 3). (B) VSMCs were treated with Ang II
(10
)7
M) for the indicated periods of time. Cell extracts were
immunoprecipitated with antibodies to c-Jun or to STAT5B and
immunoblotted with anti-phospho-Ser IgG or anti-PY99 IgG by
western blot analysis. Bar graphs show the relative level of phos-
phorylated c-Jun or phosphorylated STAT5B for four independent
experiments. *P< 0.05, compared with 0 h (n= 3). (C) VSMCs
were pretreated with or without AG490 (10
)5
M) for 16 h before
stimulation with Ang II (10
)7
M) for 3 and 6 h. Cell extracts were
immunoprecipitated with anti-STAT5B IgG and analyzed by western
blotting using anti-PY99 and anti-STAT5B IgGs, respectively. Bar
graphs show the relative level of phosphorylated STAT5B for four
independent experiments. *P< 0.05, compared with treatment
without AG490 in Ang II-treated cells for 3 and 6 h, respectively
(n= 3).
STAT5B and AP-1 interaction in AGT gene activation M. Han et al.
1722 FEBS Journal 276 (2009) 1720–1728 ª2009 The Authors Journal compilation ª2009 FEBS
A
Ang II
Nuclear extract
STAT5B probe
Cold STAT5B probe
Anti-STAT5B IgG
Anti-c-Jun IgG
Rabbit IgG
––0.5 1 3 3 – 33 3 3 3 h
–+++++ –+++++
++++++ ++++++
–––––+ –+––––
–––––– –––––+
–––––– ––––+–
–––––– –––+––
Supershift
Shift
Free probe
Ang II
Nuclear extracts
AP-1 probe
Cold AP-1 probe
Anti-c-Jun IgG
Anti-STAT5B IgG
Rabbit IgG
––0.5 1 3 3 3 –33 3 h
–++++++–+++
+++++++++++
–––––+++ –– ––
–––––––––+–
––––––––––+
––––––––+ – –
Supershift
Shift
Free probe
B
C
Ang II
AG490
–3 3 h
–– +
IP: STAT5B
No antibody
Input
Ang II 0 0.5 1 3 6 12 h
IP: c-Jun
No antibody
Input
STAT5B binding
sequence
AP-1 binding
sequence
Fig. 2. Ang II increases the DNA-binding
activity of AP-1 and STAT5B. (A and B)
VSMCs were treated with Ang II (10
)7
M)
for 0.5, 1 and 3 h. Nuclear extracts were
analyzed by EMSA using oligonucleotide
probes containing the AP-1-binding site (A)
and the STAT5B-binding site (B) in the AGT
gene promoter. Protein–DNA complexes
were separated by nondenaturing PAGE and
then visualized by autoradiography. Super-
shift assays were performed by adding anti-
bodies against c-Jun or STAT5B. Rabbit IgG
was used as negative control. The data
shown represent the best of three indepen-
dent experiments. (C) VSMCs pretreated
with or without AG490 were treated with
Ang II (10
)7
M) for the indicated periods of
time. Chromatin fragments were immuno-
precipitated by anti-c-Jun and anti-STAT5B
IgG and the AGT promoter region containing
the AP-1 ()644 to )381 bp) or the STAT5B
()200 to )60 bp) binding sequence was
amplified by PCR, respectively. The data
shown represent the best of three indepen-
dent experiments.
M. Han et al. STAT5B and AP-1 interaction in AGT gene activation
FEBS Journal 276 (2009) 1720–1728 ª2009 The Authors Journal compilation ª2009 FEBS 1723

nuclear protein with the AP-1 probe were supershifted
by antibody to STAT5B. Similarly, the STAT5B probe–
protein complexes were supershifted by antibody to
c-Jun (Fig. 2A,B). These findings indicate that AP-1
interacts with STAT5B in the AGT expression stimu-
lated by Ang II. The interaction between STAT5B and
AP-1 was also tested by cross-coimmunoprecipitation
(CoIP) of the nuclear extracts. As shown in Fig. 4A
c-Jun protein was detected in the pellets immunoprecipi-
tated with antibody to STAT5B, suggesting that
STAT5B interacts with AP-1. Treatment of VSMCs
with Ang II for 1 and 3 h resulted in an increase in the
interaction of STAT5B with c-Jun. The interaction of
STAT5B with c-Jun induced by Ang II was significantly
decreased by pretreating VSMCs with AG490, suggest-
ing that STAT5B phosphorylation is required for the
interaction of STAT5B with AP-1. To verify this further
in vivo, ChIP was performed by using antibodies to
c-Jun or to STAT5B. STAT5B protein was found
in protein eluates from anti-c-Jun IgG-precipitated
chromatin, whereas the eluates from anti-STAT5B
IgG-precipitated chromatin contained c-Jun protein
(Fig. 4B). Furthermore, ChIP assays showed that the
STAT5B-binding sequence could be amplified by PCR
in the immunoprecipitates formed with anti-c-Jun IgG,
and the AP-1-binding sequence was similarly produced
from the STAT5B–chromatin complexes immunopre-
cipitated by anti-STAT5B IgG (Fig. 4C), indicating that
STAT5B physically interacts with c-Jun by forming a
stable complex with the AGT promoter in Ang II-
induced AGT expression.
Discussion
In this report, we demonstrated, for the first time, that,
in addition to STAT5, AP-1 is an important transcrip-
tion factor which maintains the transcription of AGT
mRNA in VSMCs, and that the activation of AP-1
participates in transcription activation of the AGT gene
to modulate the autocrine Ang II loop in the local
renin-angiotensin system. Jun and Fos family proteins
usually function as dimeric transcription factors that
bind to AP-1 regulatory elements in the promoter of
numerous genes. Jun proteins can form stable homo-
dimers or heterdimers with Fos proteins. Recent study
has indicated that Ang II activates AP-1 to regulate sev-
eral inflammatory genes in VSMCs [23]. We showed
that Ang II could activate AP-1 through enhancement
of the phosphorylation and association to DNA of Jun
proteins in the induction of the AGT gene by Ang II in
VSMCs. ChIP assays confirmed that Ang II increased
the recruitment of AP-1 to the AGT gene promoter.
Overexpression of c-Jun increased AGT-Luc reporter
activity in A293 cells. These findings indicate that AP-1
activation is involved in regulatory mechanisms of
Ang II-induced AGT gene expression in VSMCs.
Ang II is known to activate the JAK-STAT pathway
in several cells [9], STAT1, STAT2 and STAT3 in
VSMCs [9,23,25,26] and STAT5 in cardiac myocytes
[8], whereas the activity of STAT5 is unknown in Ang
II-induced VSMCs under the same conditions
[9,25,27]. However, we demonstrated that Ang II
enhances the phosphorylation of STAT5B and its
association with DNA, and consequently the transacti-
vation transcription of the AGT gene in VSMCs.
Super-EMSA and ChIP confirmed that Ang II could
increase the binding activity of STAT5B to the cis-
element and the recruitment of STAT5B to the
promoter of the AGT gene in vitro and in vivo [28–32].
It was previously demonstrated that the activation
of STAT5B in the liver, and of STAT3 and STAT5A
in the heart, participates in transcription activation of
the AGT gene to modulate the autocrine Ang II loop,
and that Ang II-mediated activation of JAK2 triggers
a pattern of tissue-specific phosphorylation of the
pGL3-AGT-Luc
pcDNA3.1-STAT5B
pcDNA3.1-c-Jun
pcDNA3.1
++++
–+–+
––++
+–––
**
Relative luciferase activity
0
10
20
30
40
50
60
70
80
90
*
A
B
STAT5B
c-Jun
AP-1AP-1 STAT5 tataaa
–419~–412 –282~–277 –172~–163 TATA
+1
Fig. 3. Co-operation of STAT5B with AP-1 activates the AGT pro-
moter. (A) Schematic representation of the AP-1-binding site and
the STAT5B-binding site in the AGT promoter region. (B) 293A cells
were co-transfected with the pGL3-AGT-Luc reporter and with an
expression vector for c-Jun, STAT5B or c-Jun + STAT5B, respec-
tively. Cell lysates were subjected to luciferase activity assays and
western blotting using anti-STAT5B and anti-c-Jun IgG, respectively.
Bar graphs are expressed as the relative luciferase activity.
*P< 0.05 versus pcDNA3.1-transfected cells (n= 3).
STAT5B and AP-1 interaction in AGT gene activation M. Han et al.
1724 FEBS Journal 276 (2009) 1720–1728 ª2009 The Authors Journal compilation ª2009 FEBS


























