INTERNAL MEDECINE JOURNAL OF VIETNAM|NO 22/2021
26
CLINICAL RESEARCH
CHANGES OF CLINICAL CHARACTERISTICS IN PATIENTS
AFTER ALLOGENEIC UMBILICAL CORD-DERIVED
MESENCHYMAL STEM CELL TRANSPLANTATION
Ta Ba Thang1, Dang Thi Ngoc Quynh1, Dao Ngoc Bang1
Bach Quoc Tuan1, Nguyen Van Dung1
1Respiratory center, Military Hospital 103, Military Medical University
ABSTRACT
Objectives: To evaluate the changes of clinical and paraclinical characteristics in chronic obstructive pulmonary
disease patients after treatment of umbilical cord-derived mesenchymal stem cells. Subjects and method: 21
stable COPD patients were enrolled with C and D groups in accordance with GOLD 2018. Patients have infused
intravenously allogeneic umbilical cord-derived mesenchymal stem cells with a dosage of 1.5 X 106 MSCs
per kilogram of weight every 6 months for 6 months. Patients were followed every 3 months for 6 months:
clinical symptoms, quality-of-life (CAT), mMRC, 6-minute walking test (6MWT), some exacerbations, CRP, lung
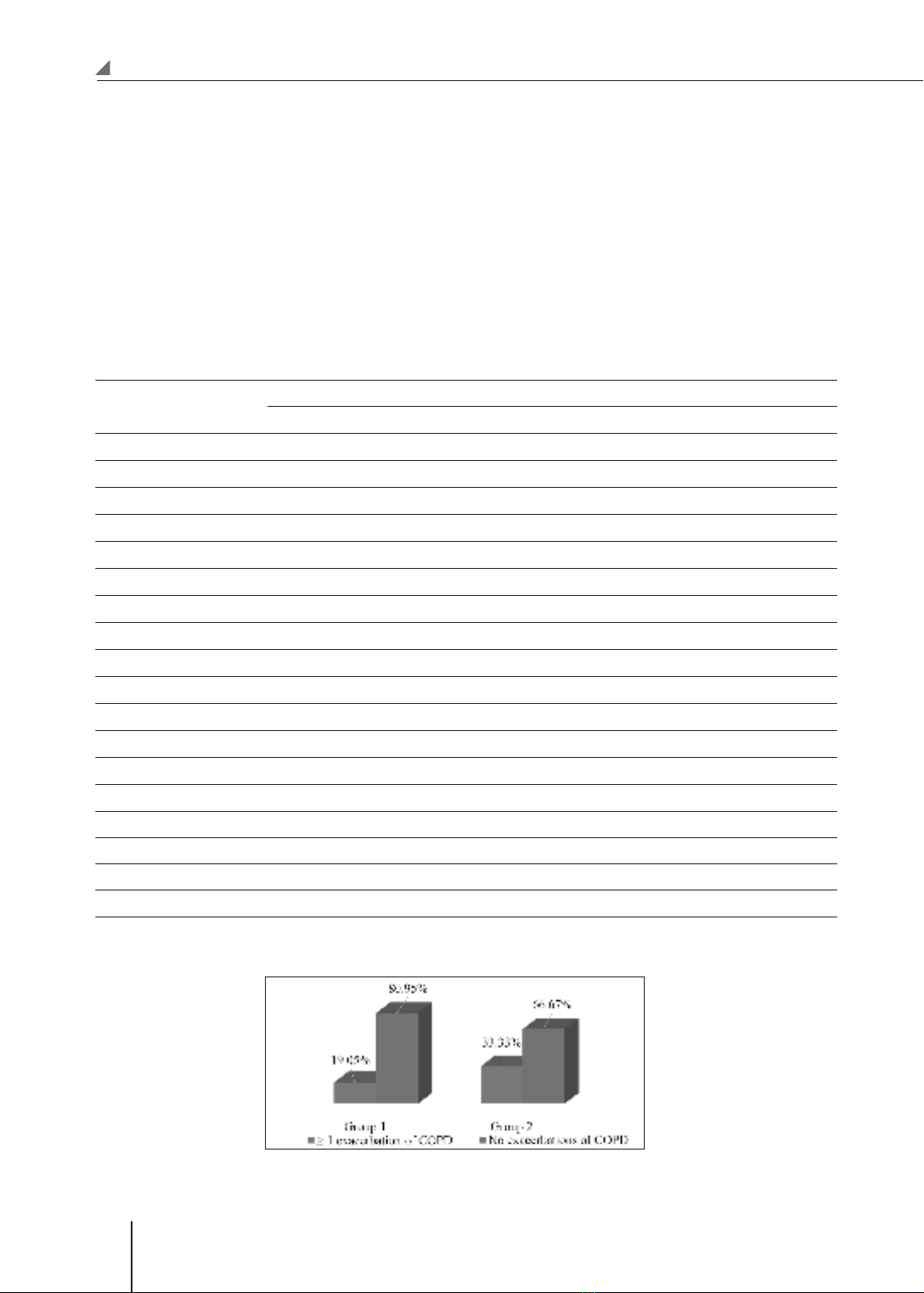
function test, and arterial blood gas. Results: After 3 months, the decrease of CAT scores, mMRC, and rate
of exacerbations in group 1 were better than that of group 2. The rate of exacerbations gradually decreased
after 1, 3, and 6 months of MSCs transplantation. Lung function had no significant change after MSCs
transplantation. Conclusions: Allogeneic MSCs administration has initially improved symptoms, quality of life,
exercise tolerance, and reduced exacerbations in COPD patients with C and D groups.
* Keywords: Allogeneic umbilical cord derived mesenchymal stem cell; Chronic obstructive pulmonary disease.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a
global burden of disease with high mortality, affecting
more than 380 million people worldwide. Current
therapies include pharmacological treatment and
non-pharmacological treatment [1, 4, 5]. However,
studies showed that recent therapies have limited
efficacy in reducing exacerbations and improving
lung function as well as in reducing mortality. Some
studies initially showed that stem cell transplantation
and lung regeneration that may be capable of
restoring pulmonary function and structures is a
potential treatment therapy in the future [2, 3, 6, 7. In
Vietnam, some hospitals have applied MSCs stem
cell transplantation in the treatment of COPD patients.
From 2018, Vietnam Military Medical University -
Military Hospital 103 applied allogeneic umbilical
cord-derived MSCs in the treatment of COPD. This
study was performed with the aim: to assess changes
in clinical and subclinical characteristics in patients
with the chronic obstructive pulmonary disease after
allogeneic umbilical cord-derived mesenchymal stem
cell transplantation.
SUBJECTS AND METHODS
1. Subjects
Including 36 patients were diagnosed with stable
COPD in groups C and D, treated in the Respiratory
Center, Military Hospital 103 from July 2018 to
March 2019, and were divided into 2 groups:
Corresponding author: Ta Ba Thang (tabathang@yahoo.com)
Date received: 06/5/2021
Date accepted: 28/5/2021