
Extraction and characterization of pectin from Yuza (Citrus junos) pomace:
A comparison of conventional-chemical and combined physicaleenzymatic
extractions
Jongbin Lim, Jiyoung Yoo, Sanghoon Ko, Suyong Lee
*
Department of Food Science & Technology and Carbohydrate Bioproduct Research Center, Sejong University, 98 Gunja-dong, Gwangjin-gu, Seoul 143-747, Republic of Korea
article info
Article history:
Received 20 May 2011
Accepted 23 February 2012
Keywords:
Yuza
Pectin
Physicaleenzymatic extraction
Rheology
Mixolab
abstract
Pectin from Yuza (Citrus junos) pomace was extracted by using combined physical and enzymatic (CPE)
treatment, and their characteristics were compared with those of chemically-extracted pectin. Their
physico-chemical and thermo-mechanical properties were also investigated in a wheat flourewater
system. The CPE extraction produced pectin with 55% of galaturonic acid and the extraction yield was
7.3%. Also, the pectin obtained by CPE extraction exhibited a higher degree of esterification (46%) than
chemically-extracted pectin (41%), which was confirmed by FT-IR analysis. When the pectin solutions
were subjected to steady-shear conditions, both samples had shear-thinning properties while high
apparent viscosity was observed in the chemically-extracted pectin. Even though the use of both pectins
raised the pasting parameters of wheat flour as well as its gelatinization temperature, less change in the
pasting properties was found in the wheat flourewater system containing the pectin prepared by CPE
treatment. The Mixolab results demonstrated that during mechanical shearing and thermal treatments,
the dough samples containing chemically-extracted pectin exhibited enhanced mixing stability, strong
protein network structure, and increased peak viscosity.
Ó2012 Elsevier Ltd. All rights reserved.
1. Introduction
Yuza belongs to citrus fruits (scientifically called Citrus junos)
and is commercially cultivated in northeast Asian countries such as
Korea, China, and Japan. Due to the uniquely tart flavor of Yuza, it is
rarely consumed as a raw fruit; its juice is mainly used as an
aromatic ingredient to produce a variety of processed foods such as
teas, drinks, and dressings. The Food and Agriculture Organization
recently reported that the total production of Yuza-processed
products in Korea has almost doubled since 2000 and also that
the preserved-Yuza market in Japan increased to 4.5 billion Yen in
2010 (FAO, 2010). Correspondingly, high amounts of Yuza pomace
(approximately 1800 ton/year) have been produced. Yuza pomace
is however disregarded as processing by-products, probably
causing environmental and economic problems for waste disposal.
Hence, there has been scientific and industrial interest in searching
for a way to utilize Yuza pomace. From a food-industrial point of
view, it would be worthwhile to utilize Yuza pomace as a source of
dietary fiber (specially, pectin) (Kim, 1999;Park, Lee, Chang, & Kim,
2001).
Extensively used as a gelling and thickening agent, pectin has
been industrially obtained under acidic conditions with oxalic
(Koubala et al., 2008), hydrochloric (Hwang, Kim, & Kim, 1998), and
sulphuric acid (Garna et al., 2007). Although the use of strong acids
provides high extraction yield and time-saving advantages, it can
cause serious environmental problems such as the disposal of
acidic wastewater and also play a negative role in consumer pref-
erence. Alternative treatments have been therefore taken into
account for minimizing the use of detrimental chemicals during
pectin extraction. Thus, several thermal and/or mechanical treat-
ments have been applied to extract pectin including ultrasound
(Panchev, Kirchev, & Kratchanov, 1988), autoclaving (Oosterveld,
Beldman, Schols, & Voragen, 2000), extrusion (Shin, Kim, Cho, &
Hwang, 2005), and subcritical water extraction (Tanaka,
Takamizu, Hoshino, Sasaki, & Goto, in press;Ueno, Tanaka,
Hosino, Sasaki, & Goto, 2008). In addition, natural catalysis with
endo-polygalacturonase (Contreras-Esquivel, Voget, Vita, Espinoza-
Perez, & Renard, 2006), (hemi)cellulase (Shkodina, Zeltser,
Selivanov, & Ignatov, 1998;Zykwinska et al., 2008), protease
(Zykwinska et al., 2008), and microbial enzymes (Ptichkina,
Markina, & Rumyantseva, 2008) have been used so far. However,
*Corresponding author. Tel.: þ82 2 3408 3227; fax: þ82 2 3408 4319.
E-mail address: suyonglee@sejong.ac.kr (S. Lee).
Contents lists available at SciVerse ScienceDirect
Food Hydrocolloids
journal homepage: www.elsevier.com/locate/foodhyd
0268-005X/$ esee front matter Ó2012 Elsevier Ltd. All rights reserved.
doi:10.1016/j.foodhyd.2012.02.018
Food Hydrocolloids 29 (2012) 160e165

there is a limited trial to extract pectin from Yuza pomace by
combined thermo-mechanical and enzymatic methods. Further-
more, pectin is shown to have a different structure and confor-
mation such as the distribution of methoxyl groups and degree of
esterification, depending on the extraction method used. This
suggests that the physico-chemical properties of pectin can be
significantly varied by the extraction method. Nonetheless, most
studies on pectin extractions have also paid primary attention to
the yield and chemical composition of extracted pectin of which
various properties were not characterized and compared with
those of the pectin extracted in a conventional chemical way.
Furthermore, comparisons have not been carried out in a food
model system for practical food applications.
In this study, pectin from Yuza pomace was extracted by
combined physical/enzymatic treatments without any use of acidic
chemicals and its physico-chemical, structural, and thermo-
mechanical properties were investigated and compared with
those of chemically-extracted pectin.
2. Materials and methods
2.1. Materials
Yuza pomace left from Yuza juice extraction was provided by
Hansung Food Co. (Jeollanam-do, Korea) and oven-dried at 80
C. It
was then ground to pass through a 100-mesh sieve. All chemicals
used in this study were of analytical grade and Viscozyme
Ò
L,
a multienzyme complex prepared from Aspergillus aculeatus,was
obtained from Novozymes (Bagsvaerd, Denmark).
2.2. Pectin extraction
Chemical and combined physical/enzymatic methods were
applied to extract pectin from Yuza pomace. In the case of the
conventional chemical method (Koubala et al., 2008), the dried
Yuza pomace powder was treated four times with 85% ethanol at
70
C for 20 min, followed by filtration with miracloth (Merck
KGaA, Darmstadt, Germany). The residue (10 g) was stirred with
oxalic acid/ammonium oxalate (0.25%, pH 4.6, 400 mL) at 85
C for
1 h and filtered with miracloth. Three volumes of 96% ethanol were
added to the filtrate and centrifuged at 14,500g for 10 min. The
precipitate was washed with 70% and 90% ethanol and then oven-
dried at 50
C for 24 h. According to the method of Min et al. (2011),
the combined physical/enzymatic treatment was also applied to
obtain pectin from Yuza pomace. The suspension of Yuza powder in
distilled water (5%, w/v) was agitated for 5 min at room tempera-
ture and filtered with miracloth. After mixing with distilled water
(5%, w/v) again, the residue was autoclaved at 121
C for 5 min,
followed by enzymatic treatment with Viscozyme
Ò
L with
1.2 10
4
fungal
b
-glucanase unit (40
C, 1 h). After boiling for
5 min to inactivate the enzyme, the suspension was dialyzed
(Spectrum Laboratories Inc., CA, USA) against distilled water for
24 h and freeze-dried.
2.3. Chemical and structural analysis
The contents of galacturonic acid and methanol in pectin were
investigated by spectrophotometric methods at 525 nm and
enzymatic treatments with alcohol oxidase, respectively (Klavons &
Bennett, 1986;Smout, Sila, Vu, Van Loey, & Hendrickx, 2005), and
the degree of esterification was obtained from the molar ratio of
methanol to galacturonic acid. Also, for the neutral sugar compo-
sition of pectin, HPAEC-PAD system (Bio-LC
Ò
, Dionex Corporation,
CA, USA) was used with a CarboPacÔPA1 column (4 250 mm,
Dionex Corporation, CA, USA) according to the method of Rascón-
Chu et al. (2009), and an FT-IR spectrometer (Nicolet Instrument
Co., Madison, WI) was employed to investigate the chemical
structure of pectin. The molecular weight distribution of pectin was
determined by high performance size exclusion chromatography
equipped with two columns in series (BioSep-SEC-S2000 and
S4000, Phenomenex Inc., CA, USA) and a refractive index detector
(Agilent 1100 series, CA, USA). Pullulans with known Mw (Shodex
standard, Showa Denko, Tokyo, Japan) were used as standards.
2.4. Rheological measurement
The flow behaviors of pectin solutions were investigated by
using a controlled-stress rheometer (AR1500ex, TA Instruments,
USA) with a 40 mm parallel plate. The solution for rheological tests
was prepared by mixing pectin with distilled water (3, 5, and 7%, w/
w) and heated at 80
C for 15 min. The pectin solutions were
subjected to steady-shearing at 25
C and the shear rates tested in
this study ranged from 1 to 500 s
1
. The reported rheological curves
were mean values of three measurements.
2.5. Thermal analysis
Thermal analysis was carried out using a differential scanning
calorimeter (DSC 200 F3 Maia, NETZSCH, Bavaria, Germany) which
was calibrated with indium (156.6
C, 28.591 J/g). Wheat flour
(5 mg) was weighed in a pan and either distilled water (25
m
L) or
pectin solution (0.5, 1.0, and 1.5%, w/w) was added. After hermet-
ically sealed, the pans were heated from 30 to 90
C at a rate of 5
C/
min and distilled water was used as reference. From the DSC curves
obtained, the peak enthalpy and temperature of starch gelatiniza-
tion were measured.
2.6. Pasting property measurement
The changes in the pasting property of wheat flour containing
pectin were investigated by using a rheometer equipped with
a starch-pasting cell (AR1500ex, TA Instruments, USA). 10.5% (w/w,
db) wheat flour slurries in 25 g of distilled water or pectin solutions
(0.5,1.0, and 1.5%, w/w) were prepared in an aluminum canister and
subjected to a programmed heating and cooling cycle where the
samples were held at 50
C for 1 min, heated to 95
Cat12
C/min,
maintained at 95
C for 2.5 min, cooled to 50
Cat12
C/min, and
allowed to stand at 50
C for 2 min.
2.7. Statistical analysis
All measurements were made in triplicate and the analysis of
variance (ANOVA) for a randomized block design was applied to
investigate the significance of difference among the samples.
Duncan’s multiple range test was then used for mean comparisons
at a confidence level of 95%.
3. Results and discussion
The yield and chemical composition of Yuza pectin extracted by
the combined physicaleenzymatic (CPE) method were investigated
and compared with those of chemically-extracted pectin. As can be
seen in Table 1, the yield of pectin extracted in a physical/enzymatic
way was determined to be 7.3%, which was slightly lower than that
of chemically-extracted pectin. It was reported that the yield of the
pectin from Yuza was typically 4e19%, depending on extraction
conditions (Park et al., 2001). It is well recognized that pectin is
composed of linear chains of galacturonic acid that are occasionally
interrupted by rhamnose and has side chains of other sugars (Shin
& Hwang, 2002). Thus, Table 1 also shows the content of
J. Lim et al. / Food Hydrocolloids 29 (2012) 160e165 161
galacturonic acid and neutral sugar in the pectins extracted by the
two different methods. The galacturonic acid content of 54.5% was
observed in the pectin obtained by CPE extraction, while
chemically-extracted pectin contained higher amounts of gal-
acturonic acid (72.3%). Thus, pectin was more effectively extracted
from Yuza pomace in a chemical way with acids, compared with
combined physical/enzymatic method. It is recognized that an
increase in acid strength (that is, decreasing pH) plays an important
role in increasing the content of galacturonic acid (Yapo, Robert,
Etienne, Wathelet, & Paquot, 2007). In addition, the physical/
enzymatic treatment could enhance the solubilization of non-
pectic compounds during pectin extraction, negatively contrib-
uting to the purity of galacturonic acid. In the case of neutral sugar,
arabinose and galactose were the main neutral sugars, representing
more than half of total neutral sugar. Moreover, rhamnose, glucose,
and xylose were included in both pectin samples. However,
compared to the pectin obtained by CPE extraction (17.6%),
chemically-extracted pectin contained less neutral sugar residues
(11.1%). Thus, greater loss of neutral sugar appeared to take place
during the acid-based extraction because neutral sugar linkage was
more susceptible to hydrolysis at low pH (Garna et al., 2007). The
combined physical/enzymatic treatment produced pectin with
a 46% degree of esterification which was 41% for chemically-
extracted pectin, thereby demonstrating that both pectins
belonged to low methoxyl pectins. According to a preceding study
(Park et al., 2001), Yuza pectin was extracted with citric, tartaric,
and hydrochloric acids, and the degree of esterification of the
extracted Yuza pectin ranged from 42 to 47%, which was favorably
compared to our results. Furthermore, it was previously reported
that enzymatic de-esterification of pectin by pectinesterase
produced blocks of carboxyl groups in the pectin backbone while
acid de-esterification was a random process (Taylor, 1982). Thus,
the low methoxyl pectins prepared by combined physical/enzy-
matic treatment might have a more blockwise distribution of
methoxyl groups because the enzyme used in this study
(Viscozyme
Ò
L) contains multiple activities including pectines-
terase (Koley, Walia, Nath, Awasthi, & Kaur, 2011).
The chemical structure of the pectin obtained by two different
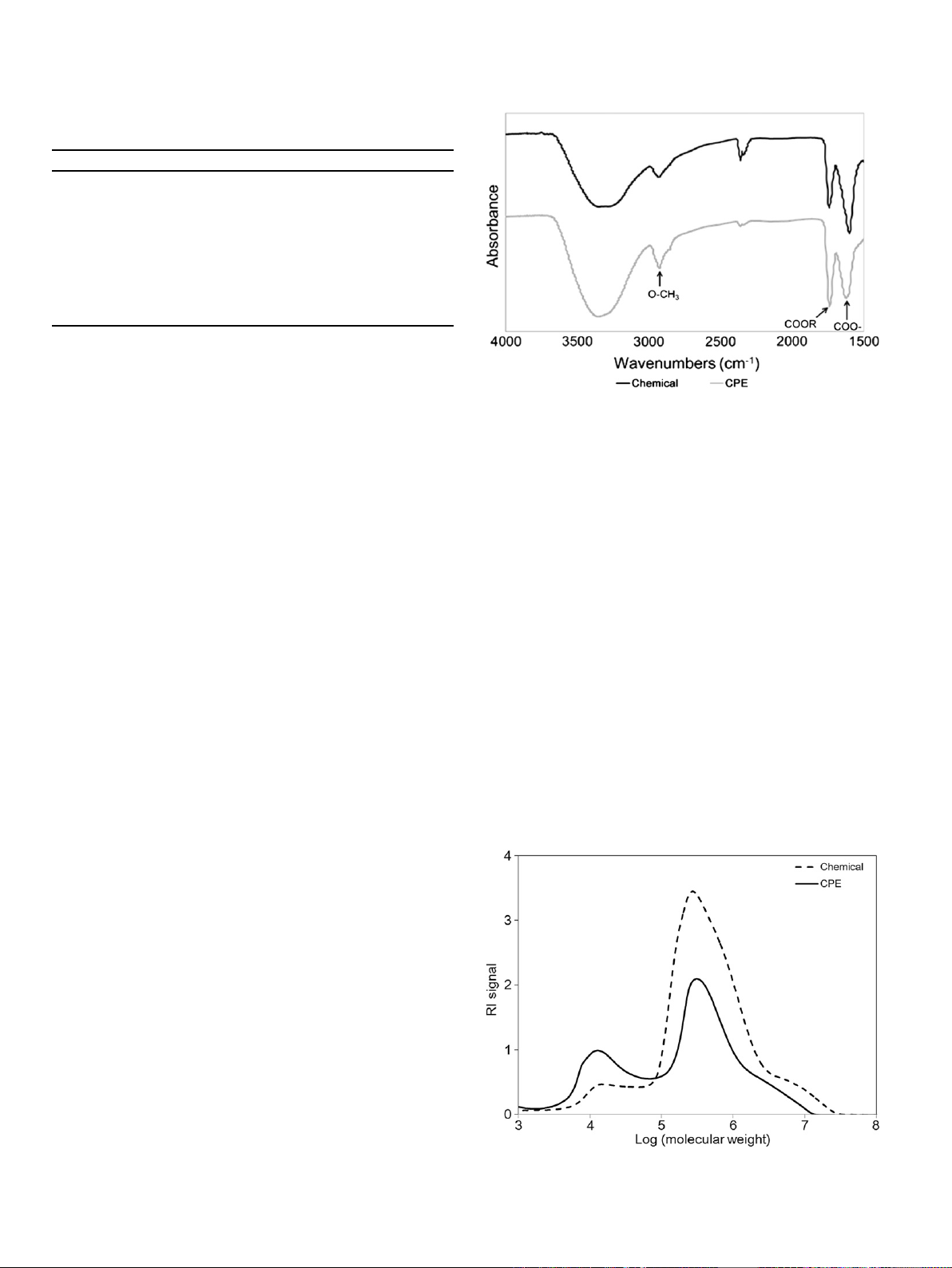
extraction methods was investigated by FT-IR. As shown in Fig. 1,an
absorption peak at 2930 cm
1
was observed for both samples,
corresponding to OeCH
3
stretching from methyl esters of gal-
acturonic acid (Liu, Cao, Huang, Cai, & Yao, 2010). Also, the carbonyl
absorption bands from free and esterified carboxyl groups were at
1620 and 1740 cm
1
, respectively (Gnanasambandam & Proctor,
2000;Liu, Cao, Huang, Cai, & Yao, 2010). However, the intensity
ratio between two peaks was distinctly different depending on the
extraction method. Specifically, the pectin extracted by CPE treat-
ments exhibited a greater intensity ratio of ester carbonyl to free
carboxyl peaks, compared to chemically-extracted pectin. It could
readily be explained by the difference in the degree of esterification
between two pectin samples. Moreover, the strong peak intensity
of total carbonyl groups was observed in chemically-extracted
pectin as expected from the content of galacturonic acid
(Table 1). Additionally, Fellah, Anjukandi, Waterland, and Williams
(2009) proposed a new method to investigate the degree of ester-
ification of pectin, which was determined from the intensity
difference between asymmetric CH
3
stretches and backbone ring
vibration.
The molecular weight distribution of pectin was investigated by
HPSEC with pullulans as standards. As shown in Fig. 2, both pectin
samples showed a major peak of molecular weight distribution
which was determined to be around 270 kDa. Also, a minor peak
(190 kDa) was observed in the lower molecular weight range which
became more pronounced in the pectin extracted by the CPE
treatment. Thus, it seemed that the chains of pectin molecules were
partially degraded by the cell wall degrading enzyme used in this
study.
Fig. 3 exhibits the flow behaviors of pectin solutions under
steady-shear conditions, which were characterized by a plot of
stress versus shear rate. Overall, they showed a convex curvature
Fig. 1. FT-IR spectra of the pectin extracted in a chemical (Chemical) or combined
physicaleenzymatic (CPE) way.
Fig. 2. Molecular weight distribution of the pectin extracted in a chemical (Chemical)
or combined physicaleenzymatic (CPE) way.
Table 1
Chemical analysis of the pectin extracted in a chemical (Chemical) or combined
physicaleenzymatic (CPE) way.
Characteristics Chemical CPE
Yield (%) 8.0 0.2 7.3 0.4
Degree of esterification (%) 41.0 0.2 46.3 0.4
Galacturonic acid content (%) 72.3 1.2 54.5 0.9
Total neutral sugar (%) 11.1 0.9 17.6 1.4
Individual neutral sugar (%)
Fucose 0.1 0.0 0.1 0.0
Rhamnose 1.1 0.2 1.4 0.2
Arabinose 5.5 0.6 10.0 0.8
Galactose 3.0 0.4 4.3 0.7
Glucose 1.3 0.6 1.0 0.3
Xylose 0.1 0.0 0.8 0.2
J. Lim et al. / Food Hydrocolloids 29 (2012) 160e165162
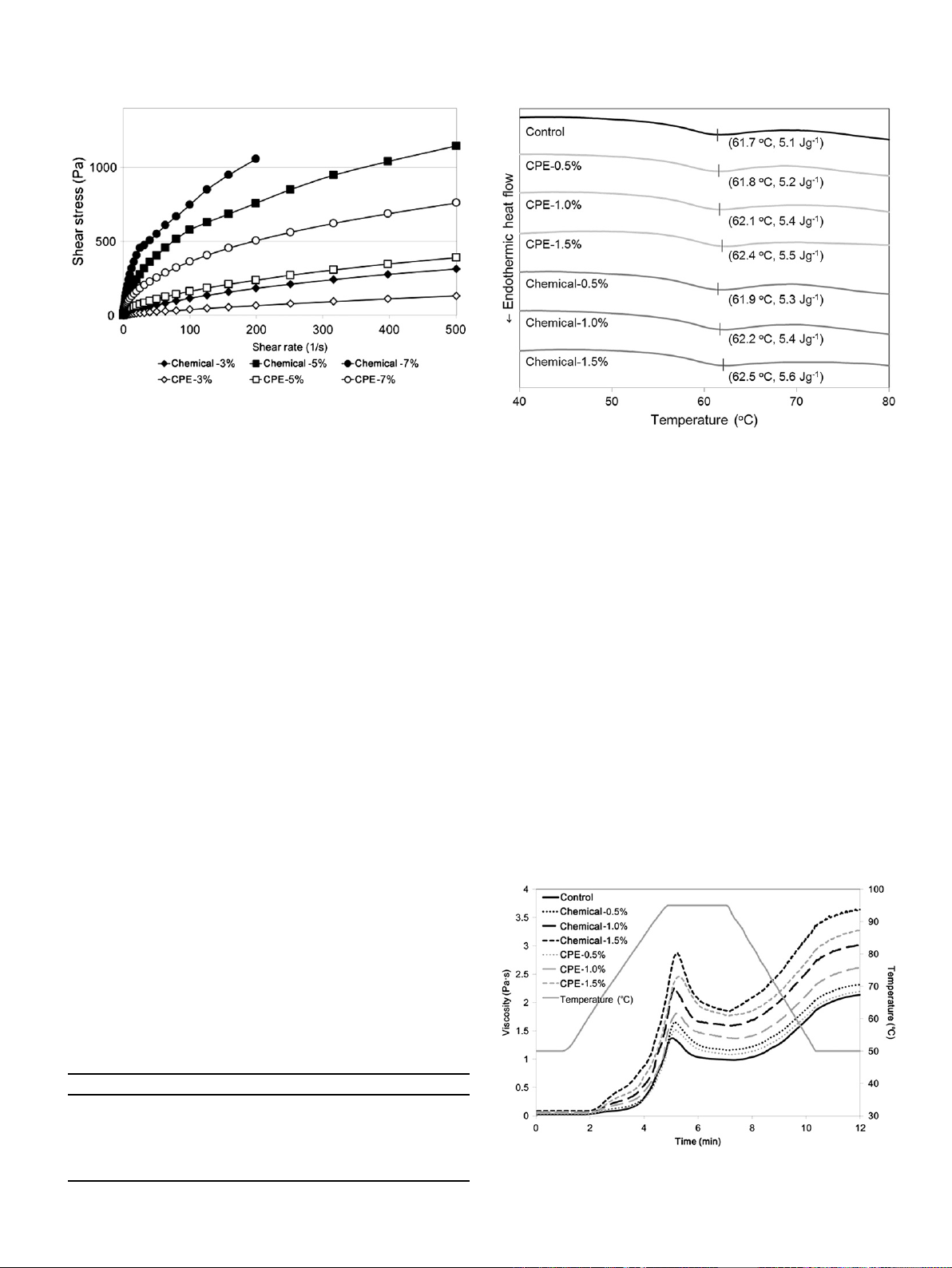
with respect to the shear rate axis, which is typical of the fluids
with shear-thinning properties. However, the chemically-extracted
pectin exhibited higher values of stress than the pectin obtained by
the CPE method at the same concentration. It indicated the higher
apparent viscosity of the chemically-extracted pectin solutions
since viscosity is equal to the ratio of shear stress to shear rate.
Moreover, these flow curves were fitted to the Power-law equation
as follows,
s
¼K_
g
n
where
s
is shear stress (Pa), _
g
is shear rate (1/s), Kis consistency
index (Pa$s
n
), and nis flow behavior index (dimensionless). As
shown in Table 2, the consistency index, corresponding to the
viscosity at a shear rate of 1 s
1
, increased with increasing pectin
concentrations. In addition, the chemically-extracted pectin had
higher consistency index. The flow behavior index was less than the
unity, confirming that all of the pectin samples had shear-thinning
properties. However, lower values of the flow behavior index
indicated that the chemically-extracted pectin exhibited more
shear-thinning characteristics, compared with the pectin extracted
in a combined physicaleenzymatic way.
The effects of pectin on the thermodynamic properties of wheat
flour were investigated as shown in Fig. 4. The peak temperature of
starch gelatinization was 61.7
C for the control wheat flour and the
temperatures of the endothermic peaks were not affected by pectin
at 0.5% level. However, greater use of pectin tended to increase the
peak temperature. This is explained by the competition for water
between starch and pectin, raising the temperature of starch
gelatinization. A slight enthalpy increase was also observed with
increasing amounts of the pectin added. However, there seemed to
be no distinct difference in the thermodynamic properties of wheat
flour with pectins from the two extraction methods.
Fig. 5 exhibits the effect of pectin on the pasting properties of
wheat flour. As can be seen in Fig. 5, all of the samples showed the
typical pasting pattern of native starch during heating and cooling
cycles epeak viscosity, breakdown, and setback. During starch
gelatinization, starch in wheat flour hydrates and swells, losing
their crystalline structure. The swollen granules are closely packed
together, consequently leading to a viscosity increase (Lii, Shao, &
Tseng, 1995). A couple of preceding studies reported that the
swelling of starch granules was decreased in the
starchehydrocolloid system, weakening the close packing struc-
ture of swollen starch granules (Biliaderis, Arvanitoyannis,
Izydorczyk, & Prokopowich, 1997). Nonetheless, the use of pectin
gave rise to a dramatic viscosity increase with increasing pectin
content. Even, the peak viscosity at a level of 1.5% increased from
1.37 Pa$s to 2.87 and 2.46 Pa$s for the pectin extracted in chemical
and physicaleenzymatic ways, respectively. As mentioned above,
starch imbibes water during starch gelatinization, making less
water available for the pectin. Therefore, it would cause an increase
in the concentration of pectin which exists in continuous inter-
granular spaces, subsequently contributing to the increased
Fig. 3. Flow behaviors of the pectin extracted in a chemical (Chemical) or combined
physicaleenzymatic (CPE) way.
Table 2
Power-law parameters of the pectin extracted in a chemical (Chemical) or combined
physicaleenzymatic (CPE) way.
Extraction method Concentration (%) KnR
2
Chemical 3 5.2 0.67 1
5 53.29 0.51 0.99
7 92.13 0.47 0.99
CPE 3 1.23 0.75 1
5 11.86 0.56 1
7 39.52 0.48 1
Fig. 4. Changes in the temperature and enthalpy of starch gelatinization by the pectin
extracted in a chemical (Chemical) or combined physicaleenzymatic (CPE) way.
Fig. 5. Changes in the pasting property of wheat flour by the pectin extracted in
a chemical (Chemical) or combined physicaleenzymatic (CPE) way.
J. Lim et al. / Food Hydrocolloids 29 (2012) 160e165 163
viscosity. Additionally, the interaction between pectin and solubi-
lized starch might partly contribute to the increased viscosity. As
can be expected from the rheological results in Fig. 3, greater
viscosity increase was observed in the samples containing
chemically-extracted pectin. Moreover, the time of the peak
viscosity was delayed by the use of pectin, thus implying that pectin
affected the starch gelatinization temperature. This was in good
agreement with the DSC results (Fig. 4) and similar trends were
reported in the previous studies (Min, Bae, Lee, Yoo, & Lee, 2010;
Rojas, Rosell, & Benedito de Barber, 1999).
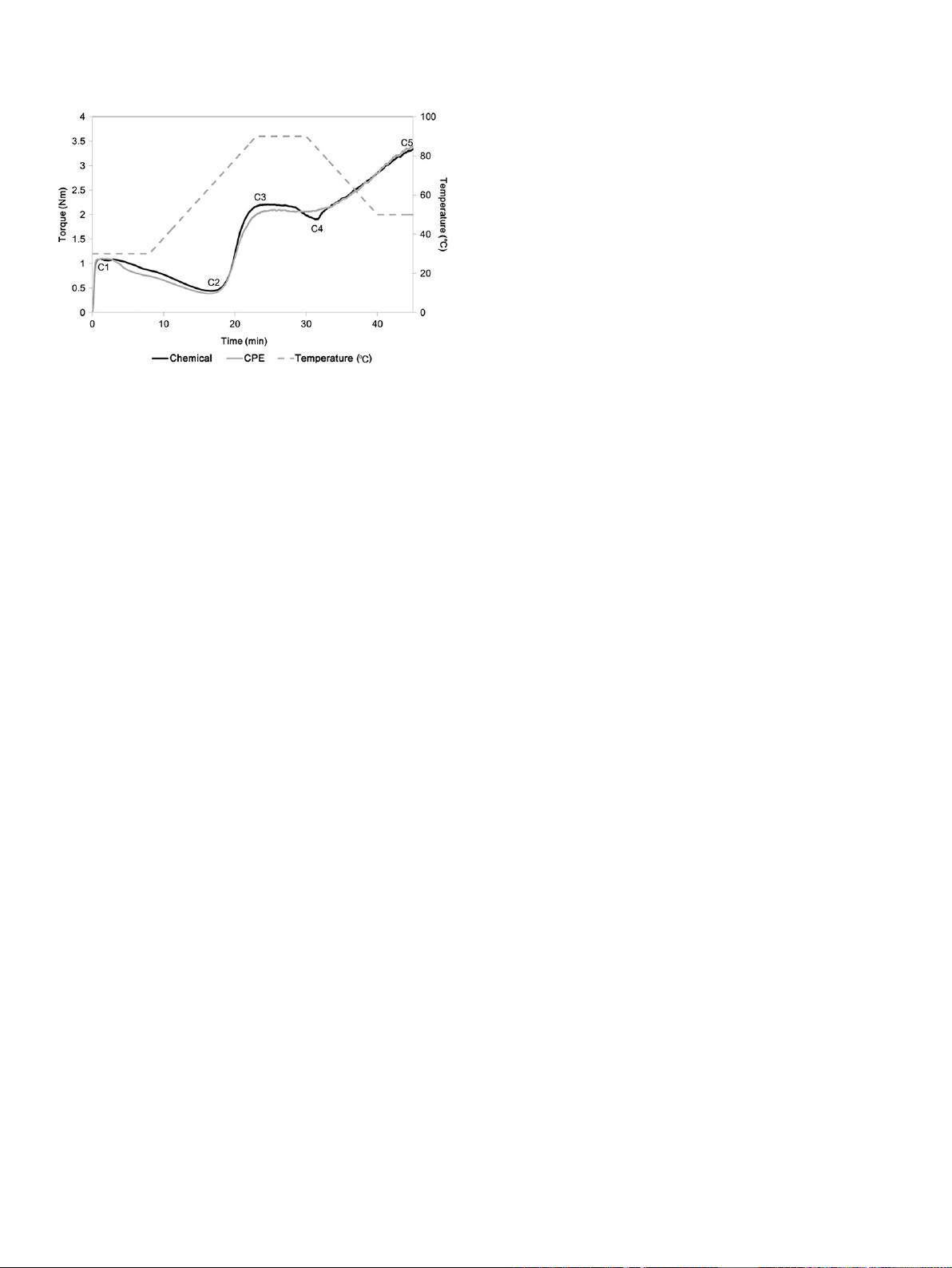
Fig. 6 exhibits the effect of pectin on the Mixolab curves of
wheat flour. In the Mixolab curve, there are five parameters to be
considered, which are maximum torque of the initial mixing stage
(C1), protein weakening (C2), starch gelatinization (C3), physical
breakdown of gelatinized starch granules (C4), and starch retro-
gradation (C5) (Kahraman et al., 2008). While the dough samples
were mixed under the condition of C1 ¼1.1 Nm, the water
absorption was 55.1% and 52.6% for the pectin obtained in chemical
and physicaleenzymatic ways, respectively. In the case of dough
stability, the use of chemically-extracted pectin produced dough
samples with greater resistance to deformation during over-mixing
as shown in the initial stage of the Mixolab curve (Fig. 6). A high
value of C2 indicated that more strong protein network was formed
in the dough containing chemically-extracted pectin. Moreover, as
manifested by C3, a greater degree of starch gelatinization could be
closely supported by the pasting property results in Fig. 5. Also, the
similar value of C5 suggests that there was no distinct difference in
the retardation of starch retrogradation in the wheat flour dough
system between both pectin samples.
4. Conclusions
Yuza pomace was subjected to combined physical and enzy-
matic treatment for producing pectin without any use of acidic
chemicals. Then, the physico-chemical, structural, and thermo-
mechanical properties of the extracted pectin were characterized
and compared with those of chemically-extracted pectin. The
results showed that low methoxyl pectin with 55% galacturonic
acid could be obtained by the combined physical/enzymatic
method without any use of chemicals, and its extraction yield was
7.3%. Both pectin solutions exhibited similar shear-thinning prop-
erties, while less viscosity was observed in the pectin obtained by
CPE treatment. In addition, when pectin was incorporated into
a wheat flour-water system, wheat flour had different patterns of
thermo-mechanical characteristics during mechanical shearing
and heating, depending on the pectin extraction method used.
This study demonstrated that pectin from Yuza pomace could be
obtained by a water-based extraction method without chemical
acids. Since consumer concerns about chemical additives have
grown, this study can contribute to the movement of the food
industry into the natural realm by eco-friendly processing tech-
nology with no harmful chemicals. This observation is also of great
significance as it contributes to process-saving and cost-effective
advantages derived from low cost treatment of wastewater.
Further efforts are needed to enhance the extraction yield and
purity of pectin and also to extend its practical use in a wider
variety of food products.
Acknowledgements
This work was carried out with the support of the “Cooperative
Research Program for Agriculture Science & Technology Develop-
ment (Project No. PJ907142)”Rural Development Administration,
Republic of Korea.
References
Biliaderis, C. G., Arvanitoyannis, I., Izydorczyk, M. S., & Prokopowich, D. J. (1997).
Effect of hydrocolloids on gelatinization and structure formation in concen-
trated waxy maize and wheat starch gels. Starch eStärke, 49,278e283.
Contreras-Esquivel, J. C., Voget, C. E., Vita, C. E., Espinoza-Perez, J. D., &
Renard, C. M. G. C. (2006). Enzymatic extraction of lemon pectin by endo-
polygalacturonase from Aspergillus niger.Food Science and Biotechnology, 15,
163e167.
FAO. (2010) FAOSTATeAgriculture Food and Agriculture Organisationof the United
Nations.
Fellah, A., Anjukandi, P., Waterland, M. R., & Williams, M. A. K. (2009). Determining
the degree of methylesterification of pectin by ATR/FT-IR: methodology opti-
misation and comparison with theoretical calculations. Carbohydrate Polymers,
78,847e853.
Garna, H., Mabon, N., Robert, C., Cornet, C., Nott, K., Legros, H., et al. (2007). Effect of
extraction conditions on the yield and purity of apple pomace pectin precipi-
tated but not washed by alcohol. Journal of Food Science, 72,C001eC009.
Gnanasambandam, R., & Proctor, A. (2000). Determination of pectin degree of
esterification by diffuse reflectance Fourier transform infrared spectroscopy.
Food Chemistry, 68,327e332.
Hwang, J. K., Kim, C. J., & Kim, C. T. (1998). Extrusion of apple pomace facilitates
pectin extraction. Journal of Food Science, 63,841e844.
Kahraman, K., Sakıyan, O., Ozturk, S., Koksel, H., Sumnu, G., & Dubat, A. (2008).
Utilization of Mixolab to predict the suitability of flours in terms of cake quality.
European Food Research and Technology, 227, 565e570.
Kim, I. C. (1999). Manufacture of citron jelly using the citron extract. Journal of the
Korean Society of Food Science and Nutrition, 28, 396e402.
Klavons, J. A., & Bennett, R. D. (1986). Determination of methanol using alcohol
oxidase and its application to methyl ester content of pectins. Journal of Agri-
cultural and Food Chemistry, 34,597e599.
Koley, T. K., Walia, S., Nath, P., Awasthi, O. P., & Kaur, C. (2011). Nutraceutical compo-
sition of Zizyphus mauritiana Lamk (Indian ber): effect of enzyme-assisted pro-
cessing. International Journal of Food Sciences and Nutrition, 62,276e279.
Koubala, B. B., Kansci, G., Mbome, L. I., Crepeau, M. J., Thibault, J. F., & Ralet, M. C.
(2008). Effect of extraction conditions on some physicochemical characteristics
of pectins from “Améliorée”and “Mango”mango peels. Food Hydrocolloids, 22,
1345e1351.
Lii, C., Shao, Y., & Tseng, K. (1995). Gelation mechanism and rheological properties of
rice starch. Cereal Chemistry, 72, 393e400.
Liu, L., Cao, J., Huang, J., Cai, Y., & Yao, J. (2010). Extraction of pectins with different
degrees of esterification from mulberry branch bark. Bioresource Technology,
101, 3268e3273.
Min, B., Bae, I. Y., Lee, H. G., Yoo, S. H., & Lee, S. (2010). Utilization of pectin-enriched
materials from apple pomace as a fat replacer in a model food system. Bio-
resource Technology, 101,5414e5418.
Min, B., Lim, J., Ko, S., Lee, K. G., Lee, S. H., & Lee, S. (2011). Environmentally friendly
preparation of pectins from agricultural byproducts and their structural/rheo-
logical characterization. Bioresource Technology, 102, 3855e3860.
Oosterveld, A., Beldman, G., Schols, H. A., & Voragen, A. G. J. (2000). Characterization
of arabinose and ferulic acid rich pectic polysaccharides and hemicelluloses
from sugar beet pulp. Carbohydrate Research, 328,185e197.
Panchev, I., Kirchev, N., & Kratchanov, C. (1988). Improving pectin technology.
International Journal of Food Science and Technology, 23,337e341.
Park, S. M., Lee, H. H., Chang, H. C., & Kim, I. C. (2001). Extraction and physico-
chemical properties of the pectin in citron peel. Journal of the Korean Society of
Food Science and Nutrition, 30, 569e573.
Ptichkina, N. M., Markina, O. A., & Rumyantseva, G. N. (2008). Pectin extraction from
pumpkin with the aid of microbial enzymes. Food Hydrocolloids, 22,192e195.
Fig. 6. Thermo-mechanical properties of wheat flour dough containing the pectin
extracted in a chemical (Chemical) or combined physicaleenzymatic (CPE) way.
J. Lim et al. / Food Hydrocolloids 29 (2012) 160e165164










![Câu hỏi ôn tập Thức ăn chăn nuôi [năm hiện tại]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250709/kimphuong1001/135x160/96231752221144.jpg)
![Đề cương ôn thi Phụ gia thực phẩm [năm hiện tại]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20251120/kimphuong1001/135x160/63671763608893.jpg)








![Đề cương ôn thi giữa kì môn Đánh giá cảm quan trong kiểm soát chất lượng [năm]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20251003/maihonghieu2004@gmail.com/135x160/69751759740815.jpg)




![Bài giảng Công nghệ chế biến và kiểm soát chất lượng thịt, thủy sản [mới nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20251001/123ngocdien/135x160/96891759397352.jpg)
