
Trehalose synthase converts glycogen to trehalose
Yuan-Tseng Pan
1
,J.D.Carroll
2
,NaokiAsano
3
, Irena Pastuszak
1
, Vineetha K. Edavana
1
andAlanD.Elbein
1
1 Department of Biochemistry and Molecular Biology, University of Arkansas for Medical Sciences, Little Rock, AR, USA
2 Department of Microbiology and Immunology, University of Arkansas for Medical Sciences, Little Rock, AR, USA
3 Faculty of Pharmaceutical Sciences, Hokurika University, Kanagawa-machi, Kanazawa, Japan
Trehalose is a nonreducing disaccharide of d-glucose
in which the two glucoses are linked in an a,a-1,
1-glycosidic linkage [1,2]. Trehalose can play a number
of different roles in biological systems, including serv-
ing as a reservoir of glucose for energy and ⁄or carbon
[3]; functioning as a stabilizer or protectant of proteins
Keywords
amylase; glycogen levels; growth on
trehalose; trehalose mutants; validoxylamine
Correspondence
A. D. Elbein, Department of Biochemistry
and Molecular Biology, UAMS, 4301 West
Markham Street, Slot 516, Little Rock, AR
72205, USA
Fax: +1 501 686 8169
Tel: +1 501 686 5176
E-mail: elbeinaland@uams.edu
(Received 5 March 2008, revised 11 April
2008, accepted 30 April 2008)
doi:10.1111/j.1742-4658.2008.06491.x
Trehalose (a,a-1,1-glucosyl-glucose) is essential for the growth of mycobac-
teria, and these organisms have three different pathways that can produce
trehalose. One pathway involves the enzyme described in the present study,
trehalose synthase (TreS), which interconverts trehalose and maltose. We
show that TreS from Mycobacterium smegmatis, as well as recombinant
TreS produced in Escherichia coli, has amylase activity in addition to the
maltose Mtrehalose interconverting activity (referred to as MTase). Both
activities were present in the enzyme purified to apparent homogeneity
from extracts of Mycobacterium smegmatis, and also in the recombinant
enzyme produced in E. coli from either the M. smegmatis or the Mycobac-
terium tuberculosis gene. Furthermore, when either purified or recombinant
TreS was chromatographed on a Sephacryl S-200 column, both MTase and
amylase activities were present in the same fractions across the peak, and
the ratio of these two activities remained constant in these fractions. In
addition, crystals of TreS also contained both amylase and MTase activi-
ties. TreS produced both radioactive maltose and radioactive trehalose
when incubated with [
3
H]glycogen, and also converted maltooligosaccha-
rides, such as maltoheptaose, to both maltose and trehalose. The amylase
activity was stimulated by addition of Ca
2+
, but this cation inhibited the
MTase activity. In addition, MTase activity, but not amylase activity, was
strongly inhibited, and in a competitive manner, by validoxylamine. On the
other hand, amylase, but not MTase activity, was inhibited by the known
transition-state amylase inhibitor, acarbose, suggesting the possibility of
two different active sites. Our data suggest that TreS represents another
pathway for the production of trehalose from glycogen, involving maltose
as an intermediate. In addition, the wild-type organism or mutants blocked
in other trehalose biosynthetic pathways, but still having active TreS, accu-
mulate 10- to 20-fold more glycogen when grown in high concentrations
(‡2% or more) of trehalose, but not in glucose or other sugars. Further-
more, trehalose mutants that are missing TreS do not accumulate glycogen
in high concentrations of trehalose or other sugars. These data indicate
that trehalose and TreS are both involved in the production of glycogen,
and that the metabolism of trehalose and glycogen is interconnected.
Abbreviations
MTase, maltose Mtrehalose interconverting activity; TPP [OtsB], trehalose phosphate phosphatase; TPS [OtsA], trehalose phosphate
synthase; TreS, trehalose synthase; TreY, maltooligosyl trehalose synthase; TreZ, maltooligosyl trehalose trehalohydrolase.
3408 FEBS Journal 275 (2008) 3408–3420 ª2008 The Authors Journal compilation ª2008 FEBS

and membranes during times of stress [4]; acting as a
regulatory molecule in the control of glucose metabo-
lism [5]; serving as a transcriptional regulator [6]; and
playing a structural and functional role as a compo-
nent of various cell wall glycolipids in mycobacteria
and related organisms [7].
In Mycobacterium smegmatis and related organisms,
there are at least three different pathways that can give
rise to trehalose [1,8]. The best known and most wide-
spread pathway in many biological systems is referred
to as the TPS ⁄TPP or OtsA ⁄OtsB pathway, which
involves two enzymes. The first enzyme, trehalose
phosphate synthase (TPS or OtsA), transfers glucose
from UDP-glucose to glucose 6-phosphate to form
trehalose phosphate and UDP [9]. The second
enzyme is a highly specific phosphatase, trehalose-
phosphate phosphatase (TPP or OtsB), that removes
the phosphate to produce free trehalose plus inorganic
phosphate [10]. A second pathway of more limited
scope in biological systems also involves two enzymes
that convert glycogen to trehalose [11]. The first
enzyme of this pathway is maltooligosyl trehalose syn-
thase (TreY), which changes the a1-4 linkage at the
reducing end of bacterial glycogen to the a,a,1,1-link-
age of trehalose. The second enzyme, maltooligosyl
trehalose trehalohydrolase (TreZ), cleaves the a1,4-gly-
cosidic linkage to which the newly-formed trehalose is
attached, producing free trehalose and leaving a glyco-
gen chain minus two glucoses [12]. The third pathway
involves a single enzyme, trehalose synthase (TreS),
which catalyzes the interconversion of maltose and
trehalose [13,14]. Although TreS can produce trehalose
from maltose, it has been postulated that its real role,
at least in corynebacteria, is to control intracellular
levels of trehalose by converting excess trehalose to
maltose, which can then be converted by a-glucosidas-
es to glucose [15,16]. By contrast, mycobacteria have a
potent trehalase [17], whereas corynebacteria do not.
Therefore, the TreS of mycobacteria may have a differ-
ent and more significant role in the synthesis of treha-
lose from maltose. However, until now, it has not been
clear where mycobacteria could obtain the maltose to
transform into trehalose because M. smegmatis grows
very poorly on maltose.
Our preliminary experiments suggested that TreS
was somehow involved in glycogen synthesis and deg-
radation. Thus, it was important to determine how the
presence of TreS affects the levels of glycogen and tre-
halose in cells. Accordingly, mutants of M. smegmatis
that were missing TreS or one of the other trehalose
biosynthetic pathways were prepared (for designation
of mutants, see Table 1) and the levels of glycogen and
trehalose were compared in these cells. In addition,
either recombinant TreS made in Escherichia coli,or
TreS purified from the wild-type M. smegmatis, was
assayed to determine its substrate specificity, and its
sensitivity to various inhibitors of trehalose or glyco-
gen metabolism. These studies demonstrated that TreS
contains amylase activity, in addition to its malt-
ose Mtrehalose interconverting activity (referred to as
MTase). These experiments also show that all of the
M. smegmatis stains that contain TreS accumulate
large amounts of glycogen when grown in high concen-
trations of trehalose, but mutants missing TreS activity
do not accumulate glycogen, regardless of the amount
of trehalose in the media. The results obtained indicate
that TreS plays an key role in the utilization of treha-
lose for the production of glycogen. We hypothesize
that TreS acts as a sensor or regulator of trehalose
levels in these cells by catalyzing the conversion of gly-
cogen to trehalose when cytoplasmic trehalose levels
are low, but this enzyme also can expedite or promote
the conversion of trehalose to glycogen when cytoplas-
mic trehalose levels become too high.
Results
Purification and demonstration of two activities
TreS was initially purified to near homogeneity from
extracts of M. smegmatis as previously described [14].
The final preparation showed one major band on SDS
gels with a molecular mass of approximately 68 kDa.
This activity of TreS, referred to here as MTase, cata-
lyzed the conversion of trehalose to maltose as mea-
sured by the reducing sugar method, or by the
formation of maltose on the Dionex carbohydrate ana-
lyzer [14]. MTase also catalyzed the reverse reaction
(i.e. the conversion of maltose to trehalose). Studies on
the substrate specificity of TreS showed that the puri-
fied enzyme could also produce maltose from either
glycogen or maltooligosaccharides (amylase activity).
This second activity was of considerable interest
because it suggested that at least one function of TreS
Table 1. Enzymatic profiles of various mycobacterial trehalose bio-
synthetic mutants.
Mutant
designation
Enzyme(s) missing
(trehalose biosynthesis)
Trehalose biosynthetic
pathways (active)
Wild-type None All (i.e. TPS ⁄TPP;
TreS TreY ⁄TreZ)
#47 TPP TreS; TreY ⁄TreZ
#74 TPS, TPP, TreY TreS
#91 TreS TPS ⁄TPP; TreY ⁄TreZ
#80 TPS ⁄TPP, TreS, TreY None
Y. T. Pan et al. TreS converts glycogen to trehalose
FEBS Journal 275 (2008) 3408–3420 ª2008 The Authors Journal compilation ª2008 FEBS 3409

could be to convert glycogen to trehalose by a series
of reactions: glycogen fimaltose Mtrehalose. Tre-
halose has been shown to be essential for the growth
of mycobacteria [18,19]; therefore, TreS could have an
important function under certain conditions, such as
when cytoplasmic trehalose levels are low, where this
enzyme could provide the essential trehalose from
glycogen.
The TreS gene from both M. smegmatis and
M. tuberculosis was cloned and expressed in E. coli
with a (His)
6
tag at the amino terminus, and active
enzyme was produced in good yield. The expressed
proteins were applied to a Ni column and the 100 mm
imidazole eluate of the column containing the purified
TreS was concentrated on the Amicon filtration appa-
ratus (Millipore, Billerica, MA, USA) several times to
remove imidazole. Both recombinant TreS prepara-
tions made from either the M. tuberculosis or the
M. smegmatis gene, as well as TreS purified directly
from extracts of M. smegmatis, undergo a self-induced
or autocatalytic proteolysis upon long-term storage on
ice, during which time the 68 kDa protein is slowly
converted to a 58 kDa protein. This transformation is
shown in Fig. 1. In this experiment, recombinant
M. smegmatis TreS, purified on the Ni column, was
kept on ice for 43 days and, at various times, samples
were removed and subjected to SDS ⁄PAGE and also
assayed for MTase and amylase activities. The MTase
activity increased as the protein was degraded and was
approximately two-fold higher in the 58 kDa protein
as in the 68 kDa MTase. On the other hand, the amy-
lase activity remained constant during this change, but
it was present in all of the intermediate proteins, as
well as in the 58 kDa protein. The 58 kDa band was
eluted from the gel and subjected to tryptic digestion
and Q-TOF MS to identify the peptides. These data
indicated that the 58 kDa protein was identical to the
68 kDa TreS, except for the loss of approximately
10 kDa of peptide from the carboxy terminus. Thus,
these data indicate that the MTase activity is increased
by the loss of the carboxy-terminal region of the pro-
tein, but the amylase activity remains at the same level
in the various intermediate forms of the enzyme.
Additional evidence that both MTase and amylase
activities reside in the same protein is demonstrated by
the experiment shown in Fig. 2. In this case, recombi-
nant M. smegmatis TreS was purified on a Ni column
and, after removal of imidazole, the protein was
allowed to remain in an ice bath for several weeks
until most of the protein had been converted to the
Fig. 1. Time course of conversion of 68 kDa TreS to 58 kDa TreS.
M. smegmatis TreS gene was cloned and expressed in E. coli with
a (His)
6
tag at the amino terminus. TreS was isolated on a Ni
column and enzyme was eluted with 100 mMimidazole. An aliquot
of the purified TreS was subjected to SDS (0.1%) ⁄PAGE (0 time),
and also was assayed for MTase and amylase activities. The TreS
elution from imidazole was stored on ice and aliquots were
removed at the times shown in the figure, and subjected to
SDS ⁄PAGE and also tested to determine the activities of MTase
and amylase. The final protein product at 43 days was mostly com-
prised of the 58 kDa band, which had both MTase and amylase
activities. The following protein standards (STD) were run on the
gels to determine the molecular weight of the TreS: rabbit muscle
myosin, 200 kDa; ß-galactosidase, 116 kDa; phosphorylase B,
97 kDa; serum albumin, 66 kDa; ovalbumin, 45 kDa; carbonic anhy-
drase, 31 kDa.
Fraction A
41–50
0.5
0.8
51–60
0.5
1.1
61–70
8
4.4
71–80
16
7.8
81–90
9.7
3.8
91–100
6.7
2.5
101–110
4.5
1.8
BC DE F GSTDs
Tubes Pooled
MTase
Amylase
Fig. 2. Gel filtration profile of 58 kDa TreS-evidence for both activi-
ties in one protein. Purified recombinant TreS prepared from the
M. smegmatis or M. tuberculosis gene was stored for several
weeks on ice to produce the 58 kDa TreS protein. This protein was
chromatographed on a 1.5 ·120 cm Sephacryl S-200 column, and
the column was eluted with 10 mMpotassium phosphate buffer
(pH 6.8), containing 1 MKCl. Fractions were collected and starting
at tube number 41, fractions were pooled in batches of ten tubes
(i.e. tubes 41–50 = fraction A; tubes 51–60 = fraction B; tubes
61–70 = fraction C; tubes 71–80 = fraction D; and so on). Fractions
were concentrated on an Amicon concentrator and an aliquot of
each fraction was subjected to SDS ⁄PAGE to identify and quanti-
tate the amount of protein, whereas another aliquot was assayed
to determine the amount of MTase and amylase activity, and the
ratios of the two. The activity of these enzymes and the ratio is
shown. Standard proteins (STDs) are as reported in Fig. 1.
TreS converts glycogen to trehalose Y. T. Pan et al.
3410 FEBS Journal 275 (2008) 3408–3420 ª2008 The Authors Journal compilation ª2008 FEBS

58 kDa form. This protein preparation was then
applied to a Sephacryl S-200 column (GE Healthcare,
Uppsala, Sweden), and fractions from the column were
collected. Starting at tube number 41, every 10 tubes
were pooled to give seven fractions as follows:
A = 41–50; B = 51––60; C = 61–70; D = 71–80;
E = 81–90; F = 91–100; G = 101–110; and
H = 111–120. An aliquot of each pooled fraction was
subjected to SDS ⁄PAGE (Fig. 2) and MTase activity
and amylase activity were also assayed in each of these
fractions. Figure 2 shows that the 58 kDa protein was
clearly evident on SDS ⁄PAGE gels in fractions C to
G, but was present in highest amounts in fractions D
and E. In addition, both MTase and amylase activities
were present in fractions B to H but, more impor-
tantly, the ratio of MTase to amylase remained fairly
constant in fractions C to F (Fig. 2, bottom). These
data strongly suggest that these two activities reside in
the same protein. As a control for these experiments,
we prepared a cell-free extract of the untransfected
vector and put it through the same purification proce-
dure. In this case, we did not find any amylase activity
in the imidazole elutions of the Ni column.
Finally, as further proof that amylase and MTase
activities reside in the same protein, we demonstrated
the presence of both activities in crystals of TreS.
These crystals had both MTase activity for converting
trehalose to maltose and amylase activity that con-
verted either glycogen or maltoheptaose to maltose
(Table 2). The amylase activity was better with malto-
heptaose as a substrate than with glycogen. A second
set of crystals was also isolated and tested in the same
way and showed both activities, although at slightly
different levels.
Demonstration of amylase activity
As described in the Experimental procedures, the
Dionex analyzer readily separates trehalose, maltose
and glucose from each other and quantifies the amount
of each sugar using an amperometric detection system.
Figure 3A shows that the amount of maltose produced
from glycogen by the recombinant TreS was linear
with time of incubation for up to 24 h, and was also
proportional to the amount of enzyme added
(Fig. 3B), for up to at least 3 lg of protein. These data
also indicate that the amylase activity was quite stable
at 37 C in the presence of glycogen because the rate
of production of maltose remained linear for at least
24 h of incubation. In these experiments, very little tre-
halose was detected at early times, probably because
the K
m
of MTase for maltose is approximately 10 mm
[14] and, therefore, even at 6 h of incubation, the
amount of maltose produced is far below the K
m
.
However, the production of trehalose from glycogen
could be demonstrated using radioactive glycogen as
the substrate, as described below.
The production of maltose from glycogen, as well as
the production of trehalose, could be demonstrated
Table 2. Enzymatic activities of MTase and amylase in crystals of
TreS. ND, not determined.
Time of
incubation
(min)
Amylase activity on [amount
of maltose (lg)]: MTase activity
[maltose
produced (lg)]
Glycogen Maltoheptaose
5 ND ND 100
10 ND ND 260
15 ND ND 288
60 1.2 2.8 ND
120 2.5 4.1 ND
480 4.0 8.6 ND
1440 0.9 14.2 ND
A
B
Fig. 3. Effect of (A) time of incubation and (B) amount of enzyme
on the production of maltose from glycogen by TreS (i.e. amylase
activity). Incubations were as described in the text and contained
0.5 mg of glycogen in 100 lLof40mMpotassium phosphate buf-
fer (pH 6.0), containing 10 mMCaCl
2
and various amounts of TreS.
The production of maltose was determined and quantitated on the
Dionex HPLC carbohydrate analyzer.
Y. T. Pan et al. TreS converts glycogen to trehalose
FEBS Journal 275 (2008) 3408–3420 ª2008 The Authors Journal compilation ª2008 FEBS 3411
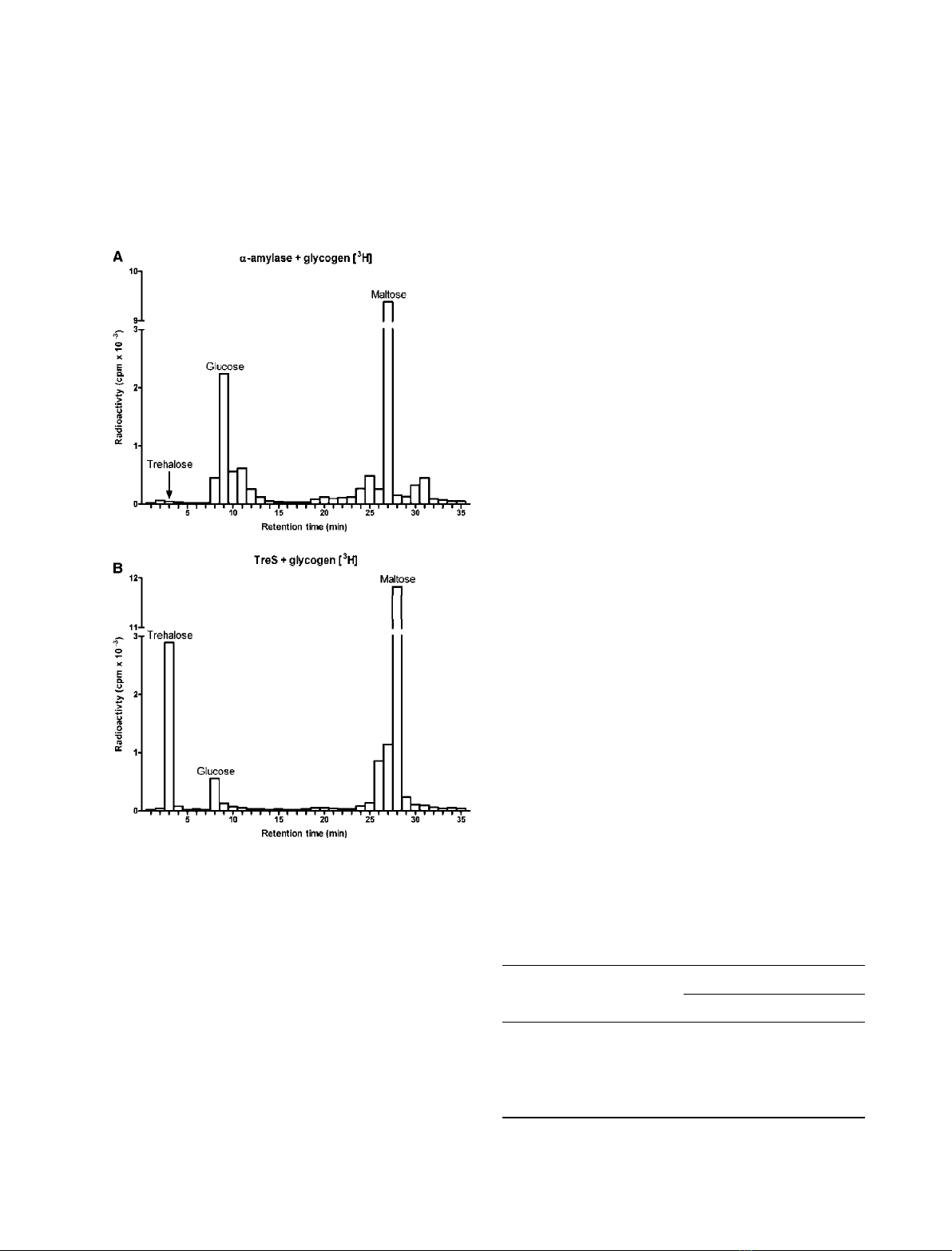
using the Dionex carbohydrate analyzer (Fig. 4).
[
3
H]glycogen was incubated either with the purified
TreS (lower profile), or with a commercial preparation
of pancreatic amylase to serve as a control (upper pro-
file). After incubation for 6 h, the reaction mixtures
were passed through a column of Biogel P-4, and those
column fractions representing the monosaccharide to
tetrasaccharide elution region of the column were
pooled, concentrated, and the radioactive sugars were
identified on the Dionex HPLC by analyzing an ali-
quot of each fraction for its radioactive content. The
upper profile shows that the pancreatic amylase gener-
ated a large peak of [
3
H]maltose and a smaller peak of
[
3
H]glucose, but no radioactive trehalose was produced
by this enzyme. By contrast, incubation with the TreS
generated a large peak of radioactive maltose as well
as a substantial peak of radioactive trehalose and a
small peak of [
3
H]glucose. The radioactive peak corre-
sponding to trehalose was completely susceptible to
digestion by a specific recombinant trehalase produced
in E. coli, and this digestion resulted in the production
of radioactive glucose as the only product (data not
shown). That maltose is the initial product produced
from glycogen was previously demonstrated by the
experiment shown in Fig. 3A when the time course
fractions were analyzed on the Dionex and essentially
no trehalose was observed at the early time points, but
was clearly evident at later times of incubation. Thus,
TreS not only has MTase activity, but also it has
amylase activity that produces the initial maltose.
Properties of the M. smegmatis amylase activity
As indicated in Fig. 3, the production of maltose from
glycogen by TreS increased in a linear fashion with
increasing time of incubation and with increasing
amounts of protein. The pH requirement for the con-
version of glycogen to maltose was determined and the
pH optimum was found to be in the range 6.0–6.2
(data not shown). Interestingly, the pH optimum for
the MTase activity (conversion of trehalose to maltose)
of TreS was previously determined to be 7.0 [14].
TreS can also use maltooligosaccharides as sub-
strates to produce maltose and then trehalose. A com-
parison of the activity of TreS on glycogen and on
maltoheptaose is presented in Table 3. Maltoheptaose
Fig. 4. Production of radioactive maltose and trehalose from
[
3
H]glycogen by TreS. [
3
H]Glycogen was incubated with either com-
mercial porcine pancreatic a-amylase (upper profile) or with purified
TreS (lower profile) for 24 h in 40 mMpotassium phosphate buffer
(pH 6.0), containing 10 mMCaCl
2
. Reactions were terminated by
heating and each mixture was passed through a 1.5 ·200 cm col-
umn of Biogel P-4. Fractions emerging in the monosaccharide
through tetrasaccharide region of the column were pooled, concen-
trated to a small volume, deionized with mixed-bed ion-exchange
resin (Dowex-1-CO
32)
and Dowex-50-H
+
) and analyzed on the Dio-
nex carbohydrate analyzer. The HPLC was equipped with a splitter
so that the fractions of the effluent could be withdrawn for deter-
mination of their radioactive content. The position of elution of the
standards glucose, maltose and trehalose are indicated on each
chromatogram and the amount of radioactivity in each area is
plotted as shown.
Table 3. Comparison of maltoheptaose and glycogen as substrates
for TreS.
Amount of substrate
(lg added to incubation)
Maltose (lg) produced from:
Maltoheptaose Glycogen
20 1.12 0.57
50 1.84 1.13
100 2.44 2.07
250 2.94 1.54
500 5.00 1.70
TreS converts glycogen to trehalose Y. T. Pan et al.
3412 FEBS Journal 275 (2008) 3408–3420 ª2008 The Authors Journal compilation ª2008 FEBS










![Hình ảnh học bệnh não mạch máu nhỏ: Báo cáo [Năm]](https://cdn.tailieu.vn/images/document/thumbnail/2024/20240705/sanhobien01/135x160/1985290001.jpg)















