
Identification of syntaxin-1A sites of phosphorylation
by casein kinase I and casein kinase II
Thierry Dubois
1
, Preeti Kerai
2,
*, Michele Learmonth
1
, Andy Cronshaw
1
and Alastair Aitken
1
1
The University of Edinburgh, Division of Biomedical and Clinical Laboratory Sciences, UK;
2
Division of Protein Structure,
National Institute for Medical Research, London, UK
Casein kinases I (CKI) are serine/threonine protein kinases
widely expressed in a range of eukaryotes including yeast,
mammals and plants. They have been shown to play a role in
diverse physiological events including membrane trafficking.
CKIais associated with synaptic vesicles and phosphory-
lates some synaptic vesicle associated proteins including
SV2. In this report, we show that syntaxin-1A is phospho-
rylated in vitro by CKI on Thr21. Casein kinase II (CKII)
has been shown previously to phosphorylate syntaxin-1A
in vitro and we have identified Ser14 as the CKII phospho-
rylation site, which is known to be phosphorylated in vivo.
As syntaxin-1A plays a key role in the regulation of neuro-
transmitter release by forming part of the SNARE (soluble
N-ethylmaleimide-sensitive factor attachment protein
receptor) complex, we propose that CKI may play a role in
synaptic vesicle exocytosis.
Keywords: CKI; CKII; syntaxin-1A; trafficking.
Casein kinase I (CKI) belongs to a family of serine/
threonine protein kinases with seven isoforms identified in
mammals (CKI a,b,d,e,c1, c2, and c3; reviewed in [1]).
The kinase domain is highly conserved between members
of the CKI family but unique N- and C-terminal tails
characterize each isoform. In yeast, the functions of CKI
have been much more extensively studied compared to
their mammalian counterparts. Recently, many reports
have linked yeast CKIs to cytokinesis and vesicle traffick-
ing especially in endocytosis [2–9]. Mammalian CKIs
appear to have similar functions and also have been
involved in DNA repair, circadian rhythms, and wnt
signaling. Like their yeast counterparts, CKIcshavebeen
implicated in cytokinesis and in membrane trafficking [10].
CKIainteracts with and phosphorylates the clathrin
adapter AP-3 [11] that is involved in endocytosis. CKIa
has been found to colocalize in neurones with synaptic
vesicle markers and to phosphorylate some synaptic vesicle
associated proteins including SV2 [12]. More importantly,
the phosphorylation of SV2 by CKI modulates its ability
to interact with synaptotagmin [13]. SV2 plays a role in
neurotransmitter release suggesting a role for CKI in this
biological process. We have recently identified centaurin-a
1
,
a protein shown to associate with presynaptic vesicular
structures [14], as a novel CKI partner [15].
In this report, we have identified syntaxin-1A as a
novel substrate for CKI, which further supports a role
for CKI in membrane trafficking. Indeed, the involve-
ment of syntaxin-1A in neurotransmitter release is well
documented (reviewed in [16,17]). Regulated neurotrans-
mitter secretion is the key step in synaptic transmission
and is the basis of intercellular communication in the
nervous system. Synaptic vesicle exocytosis is regulated
by Ca
2+
and by a large number of proteins (reviewed in
[17,18]). Syntaxin-1A is associated with the presynaptic
membrane and associates with the plasma membrane
protein SNAP-25 and the synaptic vesicle protein syna-
ptobrevin to form a ÔSNARE complexÕ.Assemblyofthis
complex is necessary and may be sufficient to trigger
membrane fusion (reviewed in [17]).
Syntaxin-1A has been previously shown to be phospho-
rylated in vitro by casein kinase II (CKII) [19–21]. Although
the site of phosphorylation was not identified, Ser14 was
speculated to be the phosphorylation site as it is present
within a CKII consensus motif. Recently, it has been shown
using phospho-specific antibodies that Ser14 is phosphory-
lated in vivo [22]. However, the kinase responsible for this
was not identified. Here, we have identified the in vitro
phosphorylation sites within syntaxin-1A that are phospho-
rylated by both CKI and CKII. CKI and CKII phospho-
rylate the N-terminal domain of syntaxin-1A on Thr21 and
on Ser14, respectively.
MATERIALS AND METHODS
Materials
[c-
32
P]ATP was from Amersham. Casein and histone H1
were purchased from Sigma. Recombinant casein kinase II
and the catalytic subunit of protein kinase A (PKA) were
from Calbiochem-Novabiochem. The plasmids encoding
the cytoplasmic domains of rat syntaxin-1A-pGEX4T-1
(1–265, 1–190 and 191–265) were obtained from T. Abe,
Niigata University, Japan. The rat munc18-1-pGEX2T
Correspondence to T. Dubois, Institut Curie – Section Recherche,
CNRS UMR 144, 26 rue d’Ulm, 75 248 Paris cedex 05, France.
Fax: + 33 1 42 34 63 77, Tel.: + 33 1 42 34 63 67,
E-mail: thierry.dubois@curie.fr
Abbreviations: CKI, casein kinase I; CKII, casein kinase II; PKA,
protein kinase A; SNARE, soluble N-ethylmaleimide-sensitive factor
attachment protein receptor.
*Present address: Wolfson Institute for Biomedical Research,
University College London, Cruciform Building, Gower Street,
London WC1E 6BT.
(Received 20 September 2001, revised 3 December 2001, accepted 4
December 2001)
Eur. J. Biochem. 269, 909–914 (2002) ÓFEBS 2002

plasmid was a kind gift from P. DeCamilli, Yale University
School of Medicine, CT, USA.
Protein purification
Recombinant GST, 14-3-3 f,14-3-3c,centaurin-a
1
and
CKIawere expressed and purified as described previously
[15,23]. Escherichia coli DH5acells containing the over-
expressing plasmid were grown overnight at 37 °Cin
500 mL Luria-Bertani broth containing 100 lgÆmL
)1
ampi-
cillin. Cultures were diluted 10-fold with fresh medium and
allowed to grow until a D
600
0.6 was reached. Expression
of GST–syntaxin-1A (1–265, 1–190 and 191–265) was
induced with 0.5 m
M
isopropyl thio-b-
D
-galactoside (IPTG,
Biogene Ltd) for 4 h at 37 °C. Bacterial cells were harvested
and resuspended in 150 mL sonication buffer (NaCl/P
i
,
1m
M
EDTA, 5 m
M
dithiothreitol, 1 m
M
benzamidine,
2lgÆmL
)1
aprotinin and 1 m
M
phenylmethanesulfonyl
fluoride) and sonicated on ice at 4 lAsixtimesfor30s
with 30-s rest intervals, using a MSE Soniprep 150 (9.5-mm
probe). The sonicate was centrifuged at 10 000 g,for
40 min at 4 °C and the supernatant was loaded onto a
NaCl/P
i
equilibrated glutathione–Sepharose matrix (Phar-
macia). The matrix was washed with 10 bed volumes of
buffer (NaCl/P
i
,500m
M
NaCl). Thrombin cleavage of
syntaxin was performed directly on the column. The column
was equilibrated with thrombin buffer (50 m
M
Tris/HCl
pH 8, 150 m
M
NaCl, 2.5 m
M
CaCl
2
). Digestion was
allowed to take place at room temperature for 1 h and
500 lL fractions were collected. Eluate containing syntaxin
was then loaded onto a benzamidine-Sepharose matrix
(Pharmacia) to remove thrombin contamination. The purity
of syntaxin was determined by analysis on 12.5% SDS/
PAGE. Syntaxin was stored in 20 m
M
Tris/HCl pH 7.5,
150 m
M
NaCl, 10% glycerol at )20 °C and protein
concentration was estimated using the Bio-Rad protein
assay. Cleavage of the GST fusion construct with thrombin
resulted in an additional five amino acids at the N-terminus
(GSPEF).
Munc18-1 was purified essentially as described for
syntaxin, except for the following. Expression of GST–
Munc18-1 was induced with 1 m
M
IPTG for 4 h at
30 °C. Munc18-1 was further purified using gel filtration
on a S-200 column. The column was washed and
equilibrated with 20 m
M
Tris/HCl pH 8, 200 m
M
NaCl.
Munc18-1 was analysed on 12.5% SDS/PAGE. Frac-
tions containing munc18-1 were stored in 20 m
M
Tris/
HClpH8,200m
M
NaCl, 10% glycerol at )70 °C.
Cleavage of the GST fusion construct with thrombin
resulted in an additional four amino acids at the
N-terminus (GSPG).
Kinase assays
Twenty picomol of purified proteins were tested for their
ability to be phosphorylated in vitro by CKIaas described
previously [15], or by CKII and PKA according to the
manufacturer’s instructions. Alternatively, 2 lgofthe
different deletion constructs of syntaxin-1A were used as
potential substrates for CKI and CKII. Reactions were
stopped by the addition of electrophoresis sample buffer
and analyzed on SDS/PAGE. Gels were stained with
Coomassie Blue and autoradiographed.
Tryptic digestion of phosphorylated proteins
Recombinant syntaxin-1A (5 lg) was phosphorylated by
CKIaor CKII. Carrier unphosphorylated syntaxin (10 lg)
was added and digested with trypsin. The peptides were
purified by reverse phase HPLC on a Vydac Ôlow TFAÕ
4.6-mm column and fractions of 0.5 mL were collected. The
elution positions of the
32
P-labelled peptides were deter-
mined by Cerenkov counting and the phosphopeptide
fractions were analysed by ion-trap mass spectrometry.
Mass spectrometry (MS) of phosphorylated peptides
Ion-trap MS of in-gel digested phosphoprotein and solid
phase sequencing on arylamine membranes were carried out
as described previously [24].
RESULTS AND DISCUSSION
Phosphorylation and dephosphorylation of protein sub-
strates by kinases and phosphatases are enzymatic activities
that play prominent roles in many biological processes
including synaptic vesicle exocytosis [17,25]. For example,
the phosphorylation of synaptic vesicle-associated protein
SV2 by CKI modulates its ability to interact with synap-
totagmin. Phosphorylation of syntaxin-1A by CKII increa-
ses its ability to interact with synaptotagmin [21]. In addition,
the phosphorylation of munc18 by PKC [26] and cdk5 [27]
leads to a significantly reduced affinity for syntaxin-1A.
A role for CKI in exocytosis has been proposed as it is
associated with synaptic vesicles and phosphorylates SV2
[12,13]. In addition, we have found that CKI interacts with
centaurin-a
1
[15], a protein that associates with presynaptic
vesicular structures [14]. Therefore, we examined whether
CKIawas capable of phosphorylating syntaxin-1A.
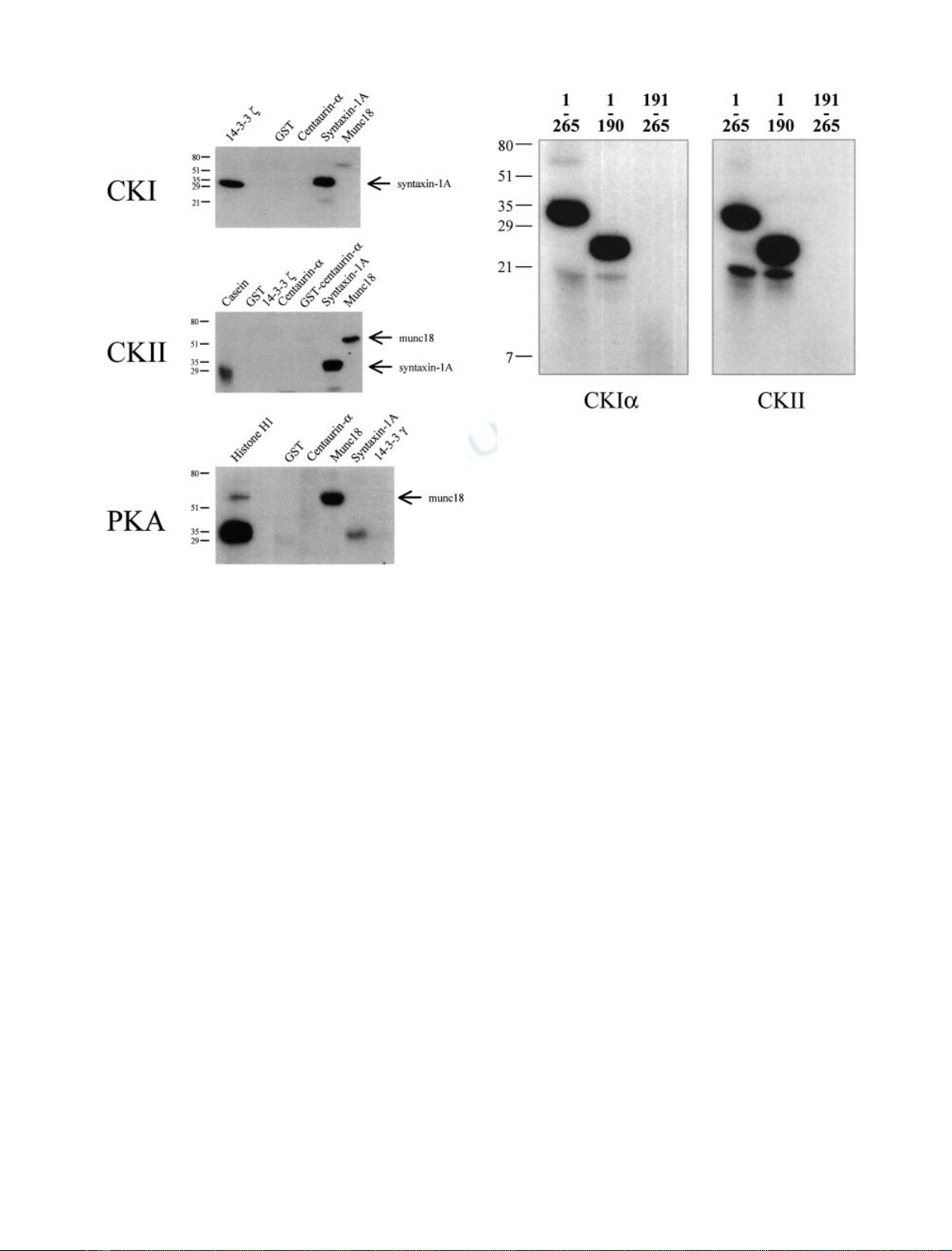
Figure 1 shows that the cytoplasmic tail of syntaxin-1A
(residues 1–265) is phosphorylated by recombinant CKIa.
Munc18, a protein which interacts with syntaxin-1A and
regulates SNARE complex formation, is not a substrate for
CKIa(Fig. 1). 14-3-3 fis phosphorylated by CKI [23] and
was used as a positive control. CKIawas unable to
phosphorylate GST or centaurin-a
1
, which were used as
negative controls [15]. Therefore, these results indicate that
CKIaspecifically phosphorylates syntaxin-1A.
In agreement with our data in Fig. 1, CKII has been
reported to phosphorylate syntaxin-1A in vitro [19–21].
GST and casein were used as negative and positive
controls, respectively. In contrast with previous reports
[20], we show that munc18 is also phosphorylated by CKII
but to a much lesser extent compared to syntaxin-1A. In
addition, we show that both centaurin-a
1
and 14-3-3 fare
not substrates for CKII, thus confirming the specificity of
the kinase activity.
Phosphorylation of syntaxin-1A and munc18 by PKA
was also investigated. Our data in Fig. 1 supports previous
reports indicating that syntaxin-1A is not a substrate for
PKA [20,21,28]. Among the potential substrates tested
(GST, centaurin-a
1,
munc18 and 14-3-3 c), only munc18
was phosphorylated by PKA. Histone H1 was used as a
positive control. In contrast to our findings, Hirling &
Scheller reported that munc18 is not a substrate for PKA
[20]. However, several potential PKA sites are present
within the primary structure of munc18 according to the
910 T. Dubois et al. (Eur. J. Biochem. 269)ÓFEBS 2002
PHOSPHOBASE
program from the Center for Biological
Sequence [29].
Figure 1 indicates that syntaxin-1A is phosphorylated by
both CKI and CKII, but not by PKA. In addition, we show
that munc18 is phosphorylated by PKA. Centaurin-a
1
,a
CKI interacting protein [15], is not phosphorylated by CKI,
CKII nor PKA.
We then identified the sites on syntaxin-1A that are
phosphorylated by CKI and CKII. Initially two truncated
mutants of syntaxin-1A (1–190 and 191–265) were subjected
to in vitro kinase assays. Syntaxin-1A (1–190), but not
syntaxin-1A (191–265), is a substrate for both kinases
indicating that the phosphorylated residue(s) are located
within the N-terminal moiety of syntaxin-1A (Fig. 2). Our
data support previous reports indicating that CKII phos-
phorylates the N-terminal 75 amino acids of syntaxin-1A
while a fragment containing residues 76–265 is not phos-
phorylated [19]. Risinger & Bennett obtained preliminary
evidence that the CKII phosphorylation site is within
residues 8–75 [21]. This region contains three potential
phosphorylation sites for CKII (Ser10, Ser14 and Thr71).
These authors reported that phosphorylation occurred on
both serine and threonine residues, thus suggesting that
Thr71 could be one of the residues phosphorylated by CKII
[21]. However our mass spectrometry results clearly show
that the peptide including this threonine is not phosphory-
lated by CKII. The peptide consisting of residues 71–84 was
identified by mass spectrometry exclusively as an unpho-
sphorylated peptide of mass 1692.9 Da (M + H)
+
.No
32
P
radioactivity was associated with this peptide from the
HPLC (data not shown).
To identify the site(s) of syntaxin-1A that are phospho-
rylated by CKIa, constructs 1–190 and 1–265 of syntaxin-1A
were phosphorylated by CKIaand subjected to trypsin
digestion. The tryptic peptides from both constructs were
separated by HPLC and the
32
P content was measured. Most
of the
32
P-labelled peptide(s) eluted in one peak (Fig. 3;
fraction 16 in syntaxin-1A). As expected, results were similar
for syntaxin-1A, 1–265 (data not shown). The radioactive
peptide peaks purified by HPLC after phosphorylation by
CKIawere analysed by ion-trap mass MS. We identified the
presence of two doubly charged peptides (M + H)
2+
,of
masses 783.7 and 824.0. The latter peptide is the phospho-
rylated form of the first one. A singly charged peptide of mass
1566.5 was also observed. These three peptides correspond to
residues 13–26 of syntaxin-1A [DSDDDDDVTVTVDR
(13–26)]. Peptides of mass 919.3 (dephospho-form) as well as
960.0 and 1917.9 Da (phospho-forms) corresponding to
residues 13–28 of syntaxin-1A [DSDDDDDVTVTVDRDR
(13–28)] were also observed due to incomplete trypsin
digestion. Tandem MS-MS sequencing of the doubly
charged peptides confirmed their identities (data not shown).
Fractions containing phosphorylated peptide(s) were ana-
lysed by solid phase sequencing. Figure 4 shows release of
32
P at each cycle of Edman degradation on covalently
coupled peptides after phosphorylation by CKIaand
purification by HPLC. The radioactivity was released in
Fig. 1. Phosphorylation of centaurin-a
1
, 14-3-3, syntaxin-1A, and
munc18 by CKI, CKII and PKA. Casein kinase I a(CKIa,upper
panel), casein kinase II (CKII, middle panel) or protein kinase A
(PKA, bottom panel) were tested for their ability to phosphorylate
in vitro 20 pmoles of centaurin-a
1
, munc18, syntaxin 1 A or 14-3-3
proteins. Histone H1, 14-3-3 fand casein were used as positive controls
for PKA, CKI and CKII, respectively. The positions of phosphory-
lated munc18 and syntaxin 1 A are indicated. The weak signals
observed with syntaxin-1A by PKA and munc18 by CKI represent
background and nonspecific phosphorylation. The positions of the
molecular mass markers (kDa) are indicated.
Fig. 2. Phosphorylation of syntaxin-1A (1–265, 1–190, 191–265) by
CKI and CKII. In order to map the CKI and CKII phosphorylation
site(s) within syntaxin 1 A, 2 lg of the cytoplasmic domain (residues
1–265) or the truncated versions (1–190 and 191–265) of syntaxin 1 A
were tested for their ability to be phosphorylated by CKIa(left panel)
or CKII (right panel). The positions of the molecular mass markers
(kDa) are indicated.
ÓFEBS 2002 Phosphorylation of syntaxin-1A by CKI and CKII (Eur. J. Biochem. 269) 911
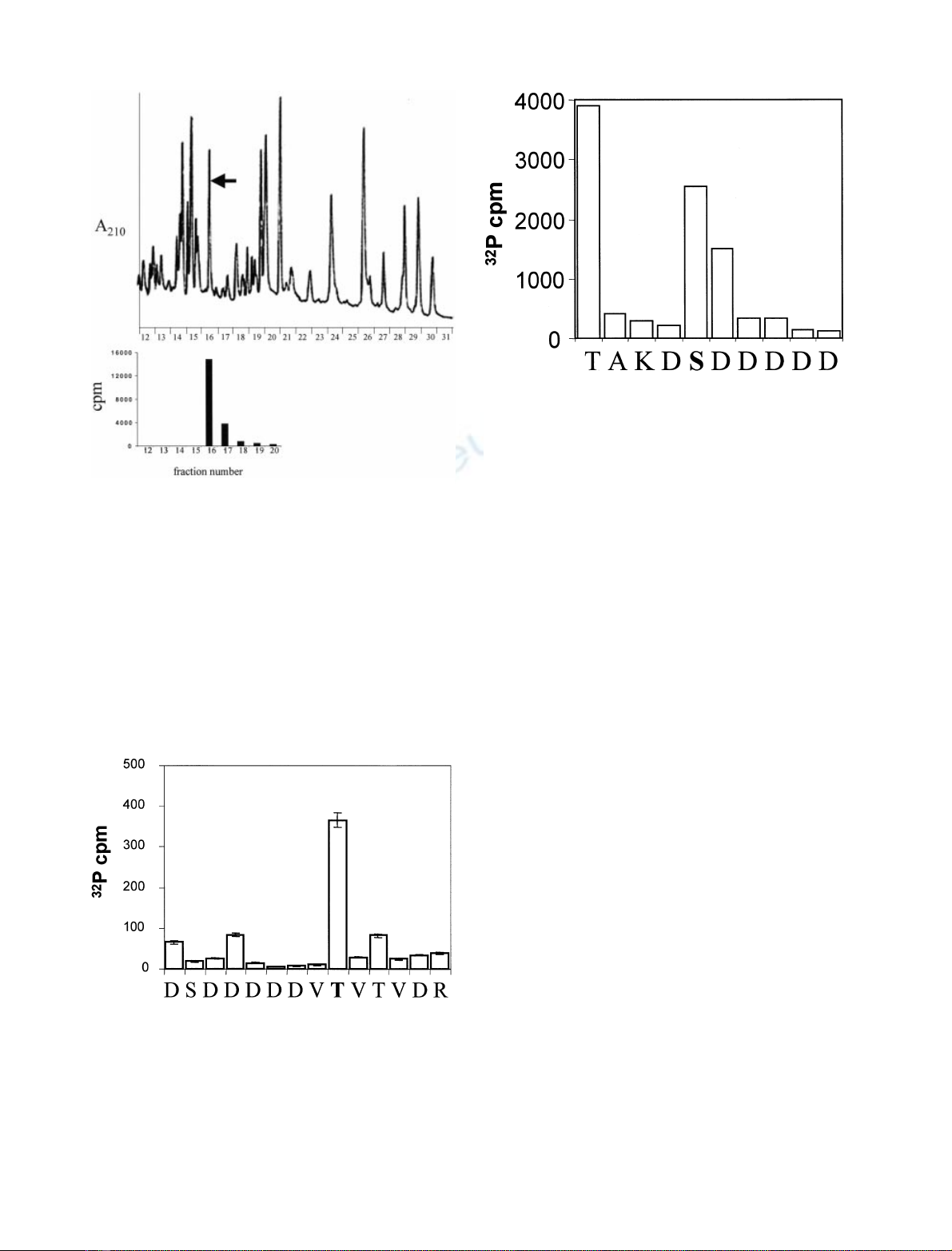
cycle 9 corresponding to residue Thr21 and this residue
represents the only amino acid phosphorylated within the
peptide. A total of three separate sequencing runs were
carried out on phosphopeptides from the two constructs.
Similarly, we identified the residue(s) of syntaxin-1A
phosphorylated by CKII. Phosphorylated forms of syn-
taxin-1A (1–190 and 1–265) were digested with trypsin and
the peptides were separated by HPLC (data not shown). The
mass spectrometry of radioactive peptides showed the
presence of a doubly charged phosphopeptide of mass
974.0 and a singly charged phosphopeptide of mass 1946.3
both of which correspond only to residues 10–26 [TAK
DSDDDDDVTVTVDR(10–26)]. We also observed a dou-
bly charged phosphopeptide of 1109.9 Da corresponding to
residues 10–28 [TAKDSDDDDDVTVTVDRDR(10–28)].
Therefore, the same region of syntaxin-1A was phosphory-
lated by both CKI and CKII. However, when phosphory-
lated by CKII, the peptide bond K12-D13 was mainly
uncleaved by trypsin because residue 14 (see results below)
was phosphorylated, and this bond was highly resistant to
trypsin cleavage. This is a consequence of residues involved
in the active site of the enzyme because trypsin readily cleaves
K/R-S
p
/T
p
bonds but normally cleaves very poorly when the
phosphoamino acid is two residues C-terminal to Arg or Lys
(i.e. K/R-X-S
p
/T
p
bonds [30]). Release of
32
P at each cycle of
Edman degradation on covalently coupled peptides after
phosphorylation of syntaxin-1A (1–265) by CKII and
purification by HPLC indicates that Ser14 (corresponding
to cycle 5) was the site of phosphorylation (Fig. 5).
Radioactivity associated with cycle 1 (Thr10) was due to
incomplete washing of the radioactivity associated nonspe-
cifically to the disc (see Fig. 6). This result indicates that there
is only one residue (Ser14) phosphorylated within the
peptide. Identical results were obtained with syntaxin-1A,
1–190 (data not shown). Two separate sequencing runs were
carried out on phosphopeptides from each of the constructs.
Because the site of phosphorylation by CKII was close to
the N-terminus of syntaxin-1A, automated Edman degra-
dation of the syntaxin (1–265) which had not been digested
with trypsin was carried out. Surprisingly this gave two
sequences in a ratio of 2 : 1 of the shorter form (Fig. 6). This
Fig. 4. CKIaphosphorylates in vitro syntaxin 1 A on Thr21. Release of
32
P at each cycle of automatic Edman degradation after phosphory-
lation of syntaxin-1A by CKIaand purification by HPLC. Peptides
(fraction 16 from Fig. 3) were covalently coupled to arylamine mem-
brane and sequenced in an ABI 477 sequencer [24]. Sequence data
from one of three runs is shown. Result indicates that the radioactivity
incorporated into the peptide is released in cycle 9 corresponding to
Thr21. This residue was the only one to be phosphorylated within the
peptide.
Fig. 3. HPLC trace (absorbance at 210 nm) with positions of
32
P-labelled tryptic peptides of syntaxin-1A (1–190) phosphorylated by
CKIa.A C-terminal truncated construct of mutant of syntaxin-1A
containing residues 1–190 was phosphorylated by CKIaand subjected
to trypsin digestion. The resulting peptides were separated by HPLC
(see the trace at A
210
nm), and the elution position of the
32
P labelled
peptide(s) was determinated by Cerenkov counting. Only one phos-
phopeptide (fraction 16) was recovered and its position is indicated by
an arrow on the HPLC trace.
Fig. 5. CKII phosphorylates in vitro syntaxin-1A on Ser14. The figure
represents the release of
32
P at each cycle of Edman degradation on
covalently coupled peptide after phosphorylation of syntaxin-1A
(1–265) by CKII and purification by HPLC. Result indicates that the
radioactivity incorporated into the peptide is released in cycle 5
corresponding to Ser14. This residue was the only one to be
phosphorylated within the peptide.
912 T. Dubois et al. (Eur. J. Biochem. 269)ÓFEBS 2002
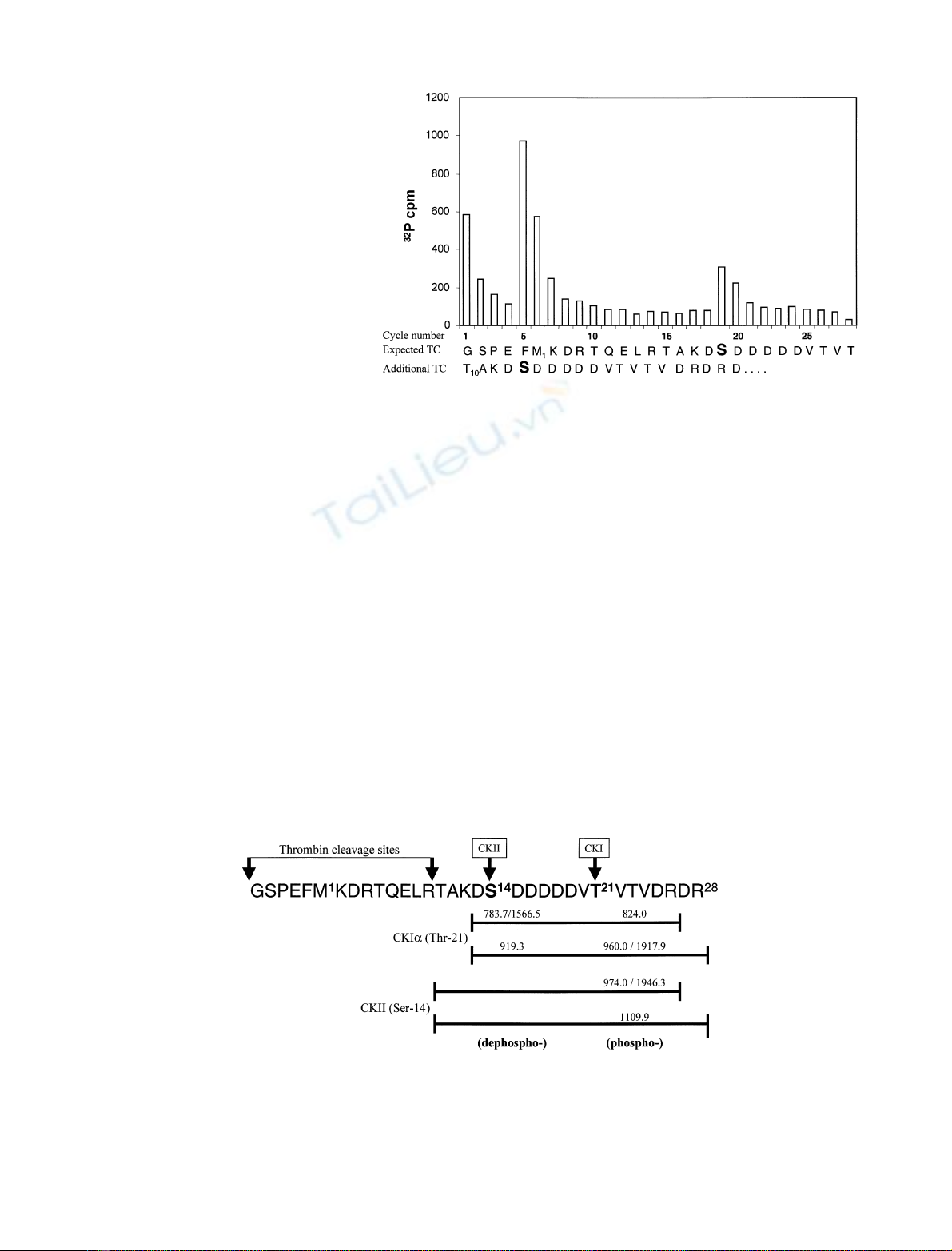
showed that the N-terminus was cleaved by thrombin to
give the main sequence of the recombinant protein (begin-
ning at residue 10; TAKDSDDDD…)aswellassome
protein cleaved at the expected thrombin cleavage site
(GSPEFM
1
KDR…). The sequence around this region of
the N-terminus would be exposed to the thrombin used to
cleave the GST fusion moiety and although it may not
appear so at first glance, it clearly must sufficiently resemble
the consensus cleavage site to be cleaved by thrombin which
has a preference for R-X peptide bonds.
Thrombin cleavage site, LVPR/GSPEFM
1
KDR…;
additional cleavage observed, QELR/TAKDSDDDD
…(where T is T10).
The direct sequencing by Edman degradation of the
protein showed a burst of radioactivity at cycle 5 which
corresponds to Ser14 (from the additional thrombin
cleavage site). No counts were observed at cycle 15 (i.e.
Thr10) verifying that the
32
P released at cycle 1 (Fig. 5) was
due to incomplete washing of nonspecific radioactivity
associated with the disc. A ÔburstÕof counts was also
observed at cycle 19 corresponding to Ser14 from the
sequencing of the protein without the additional cleavage
site (Fig. 6).
The sites of phosphorylation by CKIa(Thr21) and CKII
(Ser14) on syntaxin-1A are summarized in Fig. 7 with the
masses of the peptides identified.
To summarize, we have shown that CKIaand CKII
phosphorylate syntaxin-1A on Thr21 and on Ser14,
respectively. Both sites are located at the N-terminal domain
of syntaxin-1A. These sites are remote from the H3 domain
required for protein–protein interactions, and known to be
crucial in the formation of the four-helix bundle structure
that it is part of the SNARE complex involved in the fusion
process of synaptic vesicles with the plasma membrane.
However, the phosphorylation of syntaxin-1A by CKII
enhances its capacity to associate with synaptotagmin [21].
Therefore, phosphorylation of Ser14 by CKII suggests an
important role for this residue in regulating the interaction
between syntaxin-1A and synaptotagmin. Consistent with
our data, Foletti and coauthors have shown using specific
phosphopeptide-antibodies that Ser14 is phosphorylated
in vivo [22]. When phosphorylated on Ser14, syntaxin-1A is
preferentially associated with SNAP-25 and does not
localize with pools of synaptic vesicles [22]. Although we
have shown that CKII phosphorylates in vitro syntaxin-1A
on Ser14, and that this residue is phosphorylated in vivo,it
remains to be determined whether CKII is the kinase
Fig. 7. Summary of phosphorylation sites on recombinant syntaxin-1A. The figure shows the sites of phosphorylation by CKIa(Thr21) and CKII
(Ser14). The masses of the major peptides, in the positive ion mode (M + H)
+
, identified by mass spectrometry are indicated. The main peptides
recovered after trypsin digestion of syntaxin-1A phosphorylated by CKIaand CKII are indicated by the bars. Where two masses are shown these
are the doubly then singly charged unphosphorylated peptides followed by the masses of the corresponding phosphoforms (at 40 and 80 Da higher,
respectively). The theoretical masses are 783.8, 1566.6, 823.8, 919.4, 959.4, 1917.8, 973.9, 1946.8 and 1109.5, respectively. The two thrombin
cleavage sites are also indicated.
Fig. 6. Solid phase Edman sequencing of
syntaxin-1A (1–265) after phosphorylation by
CKII. The cytoplasmic domain of syntaxin-
1A (residues 1–265) was phosphorylated
in vitro by CKII, and automated Edman
degradation was carried out without prior
trypsin digestion. The
32
P-radioactivity and
the PTH-amino acids released at each cycle
(Ôcycle numberÕ) are shown. The top
sequence (ÔExpected TCÕ, for expected
thrombin cleavage) corresponds to the
ÔintactÕprotein after thrombin digestion. The
bottom sequence corresponds to the shorter
protein obtained from the additional
thrombin cleavage site (ÔAdditional TCÕ).
ÓFEBS 2002 Phosphorylation of syntaxin-1A by CKI and CKII (Eur. J. Biochem. 269) 913










![Hình ảnh học bệnh não mạch máu nhỏ: Báo cáo [Năm]](https://cdn.tailieu.vn/images/document/thumbnail/2024/20240705/sanhobien01/135x160/1985290001.jpg)















