PGS TS Châu Ngọc Hoa
Bộ môn Nội ĐHYD Tp HCM
Suy tim mạn:
GÓC NHÌN TỪ ACC 2017
ACC Focused update on HF, 2017

Two New Pharmacological Therapies
Approved by FDA for Heart Failure
• Ivabradine (April 15, 2015)
• Sacubitril/Valsartan (July 7, 2015)

WHO, WHEN AND
ON IVABRADINE WHY ADD
Ivabradine approval timeline
• 2005 approved in EU for angina
• 2012 approved in EU for heart failure
• 2015 approved in US for heart failure
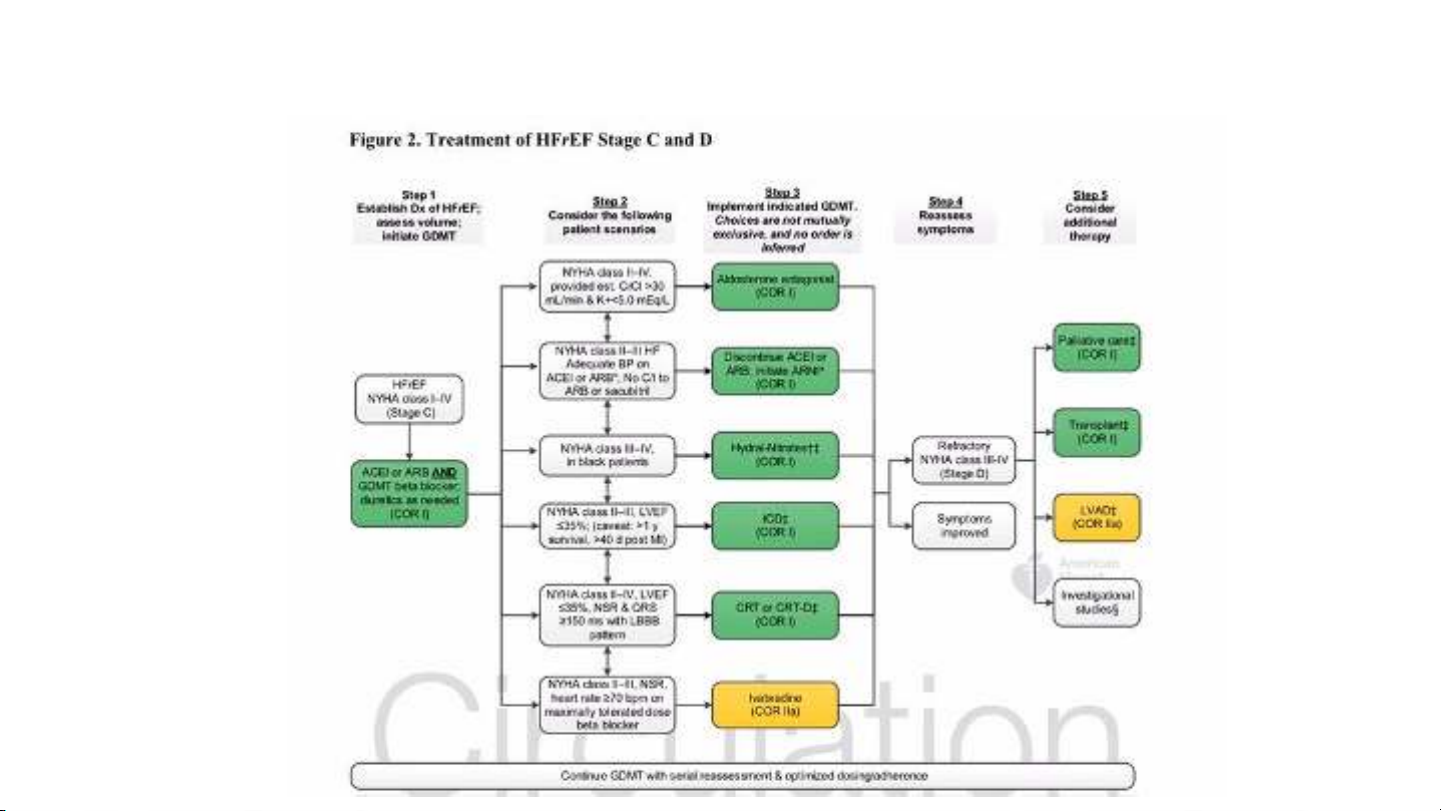
to reduce the risk for hospitalization for worsening heart failure in patients with
stable, symptomatic chronic heart failure with LVEF ≤35%, who are in sinus rhythm
with a resting heart rate of ≥70 beats per minute (bpm) and are taking maximally
tolerated doses of beta-blockers or have a contraindication to beta-blockers.









![Kỹ thuật trồng cây thuốc [chuẩn nhất]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250427/tukhauquantuong1011/135x160/7371745771325.jpg)
















