1
C H C L NG T NÂNG CAOƠ Ọ ƯỢ Ử
1- Hi u ng Zeemanệ ứ
2- Tách v ch siêu tinh tạ ế
3- S nhi u lo n ph ự ễ ạ ụ
thu c th i gianộ ờ
Ch ng b n: Các ng d ng CHLTươ ố ứ ụ
1
3
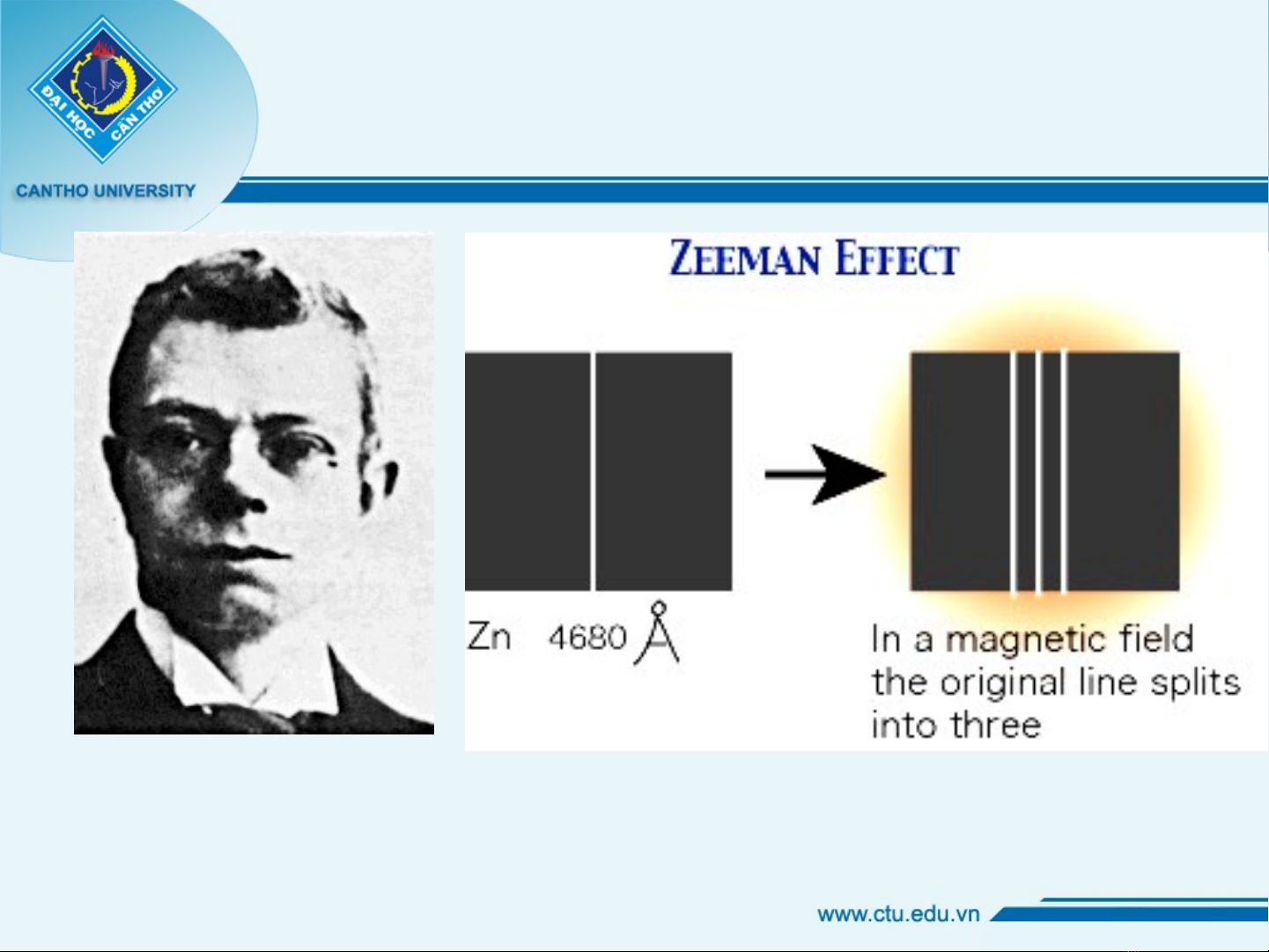
1. What is Zeeman effect?
Chân dung pietez Zeeman
3
4
1- Hi u ng Zeemanệ ứ
•Khi nguyên t đ t trong t tr ng ngoài (xem là ử ặ ừ ườ
đ u và theo ph ng 0z), ề ươ do t ng tác v i ươ ớ
momen t spin và momen t qu đ o c a ừ ừ ỹ ạ ủ
electron v i Bớex (t tr ng ngoài) làm các m c ừ ườ ứ
năng l ng c a electron b l ch đi g i là hi u ượ ủ ị ệ ọ ệ
ng Zeeman.ứ
•Hi n t ng này nh h ng đ n s ệ ượ ả ưở ế ự thay đ i ổ
b c sóng quang phướ ổ phát x c a electron.ạ ủ
•Ta gi i h n kh o sát hi u ng Zeeman ch cho ớ ạ ả ệ ứ ỉ
electron đ n ch u tác đ ng c a toán t nhi u ơ ị ộ ủ ử ễ
lo n H’ạ là năng l ng t ng tác c a momen t ượ ươ ủ ừ
spin và momen t qu đ o electron v i Bừ ỹ ạ ớ ex : 4
5
Zeeman theory
•In most atoms, there exist several
electron configurations with the same energy
so that transitions between these configurations
and another correspond to a single spectral line.
•The presence of a magnetic field breaks this
degeneracy
•since the magnetic field interacts differently with
electrons with different quantum numbers, slightly
modifying their energies.
•The result is that, where there were several
configurations with the same energy,
•They now have different energies, giving rise to
several very close spectral lines.












![Bài tập Vật lý sóng: Tổng hợp bài tập 6 [kèm lời giải chi tiết]](https://cdn.tailieu.vn/images/document/thumbnail/2025/20250805/oursky04/135x160/401768817575.jpg)














